Stereoisomers are molecules sharing the same molecular formula and sequence of bonded atoms but differ in the three-dimensional arrangement of their atoms. These variations impact physical and chemical properties, influencing drug effectiveness and material behavior. Discover how understanding stereoisomers can enhance your grasp of molecular chemistry in the rest of this article.
Table of Comparison
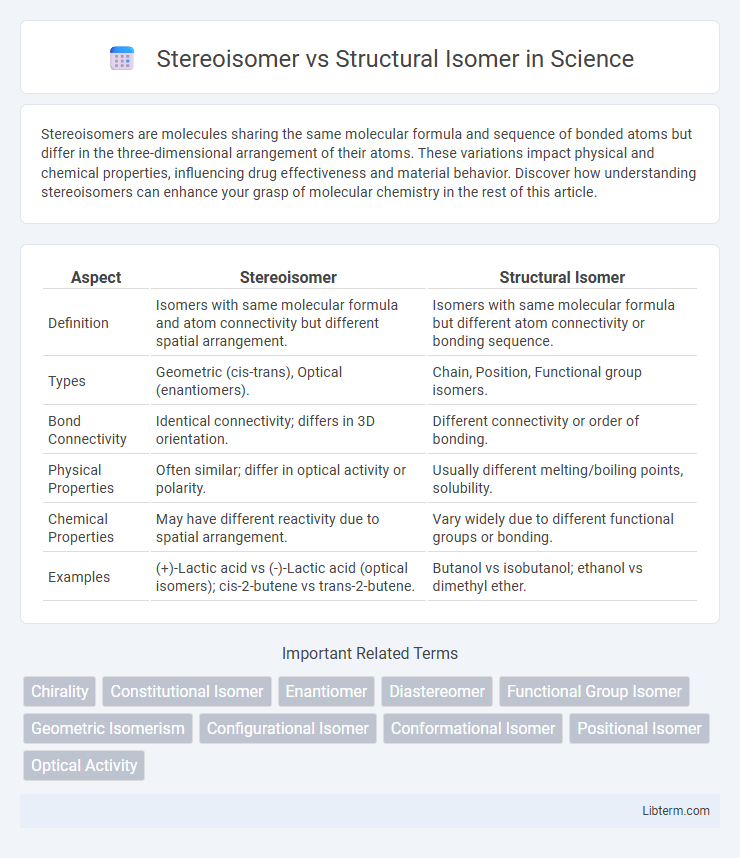
| Aspect | Stereoisomer | Structural Isomer |
|---|---|---|
| Definition | Isomers with same molecular formula and atom connectivity but different spatial arrangement. | Isomers with same molecular formula but different atom connectivity or bonding sequence. |
| Types | Geometric (cis-trans), Optical (enantiomers). | Chain, Position, Functional group isomers. |
| Bond Connectivity | Identical connectivity; differs in 3D orientation. | Different connectivity or order of bonding. |
| Physical Properties | Often similar; differ in optical activity or polarity. | Usually different melting/boiling points, solubility. |
| Chemical Properties | May have different reactivity due to spatial arrangement. | Vary widely due to different functional groups or bonding. |
| Examples | (+)-Lactic acid vs (-)-Lactic acid (optical isomers); cis-2-butene vs trans-2-butene. | Butanol vs isobutanol; ethanol vs dimethyl ether. |
Introduction to Isomerism
Isomerism refers to the phenomenon where compounds have the same molecular formula but different arrangements of atoms, resulting in distinct properties. Structural isomers differ in the connectivity of atoms, while stereoisomers share the same connectivity but differ in spatial orientation. Understanding stereoisomerism and structural isomerism is essential for grasping molecular diversity and its effects on chemical behavior.
What Are Structural Isomers?
Structural isomers are compounds with the same molecular formula but different connectivity of atoms, resulting in distinct physical and chemical properties. These isomers include chain isomers, position isomers, and functional group isomers, each varying in the arrangement of the carbon skeleton or the location of functional groups. Unlike stereoisomers, structural isomers differ in the sequence of atom bonding rather than spatial orientation.
Definition of Stereoisomers
Stereoisomers are molecules with the same molecular formula and sequence of bonded atoms but differ in the three-dimensional arrangement of atoms in space. Unlike structural isomers, which differ in the connectivity of atoms, stereoisomers include enantiomers and diastereomers that share structural frameworks yet exhibit distinct spatial orientations. Understanding stereoisomerism is crucial in fields like pharmaceuticals, where the 3D configuration impacts drug efficacy and interaction.
Key Differences: Structural vs Stereoisomers
Structural isomers differ in the connectivity of atoms within a molecule, resulting in variations in the skeleton or functional groups, while stereoisomers share the same atomic connectivity but differ in the spatial arrangement of atoms. Key differences include structural isomers having distinct molecular formulas and physical properties, whereas stereoisomers exhibit variations such as cis-trans configurations or optical isomerism without altering the molecular formula. Understanding these differences is crucial in fields like organic chemistry and pharmaceuticals, where molecular structure impacts compound behavior and biological activity.
Types of Structural Isomers
Structural isomers differ in the connectivity of atoms, with main types including chain isomers, where carbon skeletons vary; positional isomers, involving different functional group locations; and functional group isomers, where compounds have distinct functional groups but identical molecular formulas. These variations lead to unique physical and chemical properties despite sharing the same molecular formula. Understanding these types is crucial for applications in organic synthesis and pharmaceutical design.
Types of Stereoisomers
Stereoisomers include enantiomers and diastereomers, distinguished by their spatial arrangement despite identical molecular formulas. Enantiomers are non-superimposable mirror images, often differing in optical activity, while diastereomers have multiple stereocenters and differ at one or more chiral centers but are not mirror images. Cis-trans isomers and conformers are also types of stereoisomers, based on restricted rotation and conformational differences, respectively.
Methods for Identifying Isomers
Nuclear Magnetic Resonance (NMR) spectroscopy is a key technique for distinguishing stereoisomers by analyzing differences in chemical environments and spatial arrangements of atoms. Infrared (IR) spectroscopy aids in identifying structural isomers by detecting variations in functional groups and bond connectivity patterns. Chromatographic methods such as Gas Chromatography (GC) and High-Performance Liquid Chromatography (HPLC) separate isomers based on polarity and molecular interactions, providing complementary data for isomer identification.
Importance of Isomerism in Chemistry
Isomerism, including stereoisomers and structural isomers, is crucial in chemistry because it explains how molecules with the same molecular formula can have different properties and biological activities. Structural isomers differ in the connectivity of atoms, affecting physical and chemical traits, while stereoisomers have the same connectivity but differ in spatial arrangement, influencing reactivity and interaction with biological systems. Understanding these differences enables chemists to design specific drugs, materials, and chemical reactions with desired characteristics.
Real-World Examples of Isomers
Stereoisomers occur in compounds like glucose, where D-glucose and L-glucose have the same molecular formula but differ in spatial arrangement, affecting biological activity. Structural isomers include compounds such as butanol and isobutanol, which have the same molecular formula (C4H10O) but differ in connectivity, influencing their boiling points and solubility. These differences illustrate the distinct chemical and physical properties driven by isomeric variations in pharmaceuticals, food chemistry, and materials science.
Summary: Structural vs Stereoisomer Comparison
Structural isomers differ in the connectivity of their atoms, resulting in unique molecular formulas and distinct physical and chemical properties. Stereoisomers maintain the same atomic connectivity but vary in spatial arrangement, including enantiomers and diastereomers that impact properties like optical activity. Understanding the differences between structural and stereoisomers is crucial for applications in chemistry, pharmaceuticals, and materials science.
Stereoisomer Infographic
 libterm.com
libterm.com