Colloids are mixtures where tiny particles are dispersed throughout a continuous medium without settling out. These particles, ranging from 1 to 1000 nanometers, create unique properties such as opalescence and stability that differ from true solutions or suspensions. Explore the rest of the article to understand how colloids impact various scientific and industrial applications relevant to your needs.
Table of Comparison
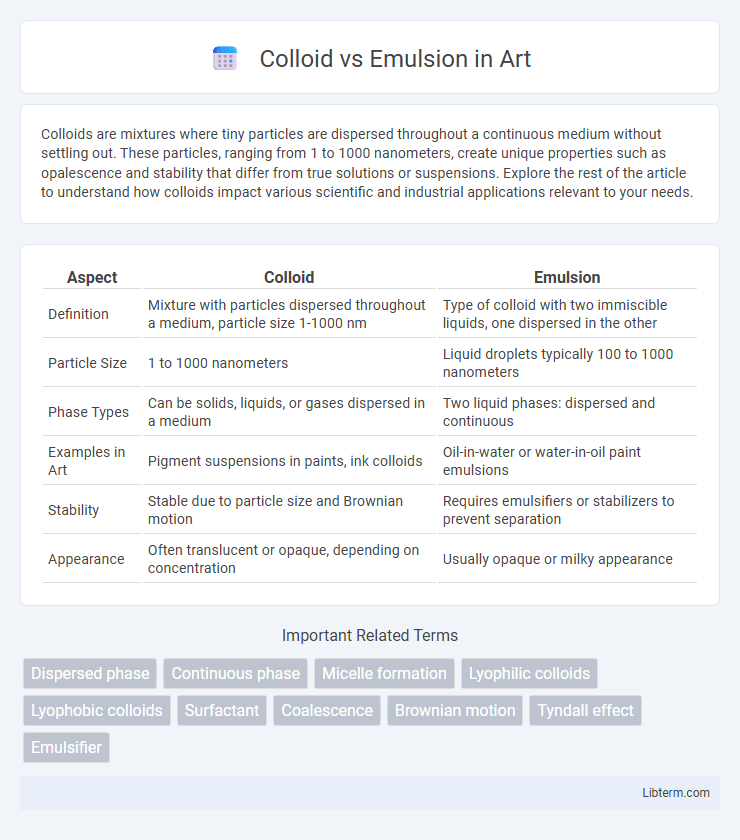
| Aspect | Colloid | Emulsion |
|---|---|---|
| Definition | Mixture with particles dispersed throughout a medium, particle size 1-1000 nm | Type of colloid with two immiscible liquids, one dispersed in the other |
| Particle Size | 1 to 1000 nanometers | Liquid droplets typically 100 to 1000 nanometers |
| Phase Types | Can be solids, liquids, or gases dispersed in a medium | Two liquid phases: dispersed and continuous |
| Examples in Art | Pigment suspensions in paints, ink colloids | Oil-in-water or water-in-oil paint emulsions |
| Stability | Stable due to particle size and Brownian motion | Requires emulsifiers or stabilizers to prevent separation |
| Appearance | Often translucent or opaque, depending on concentration | Usually opaque or milky appearance |
Introduction to Colloids and Emulsions
Colloids are mixtures where tiny particles, ranging from 1 to 1000 nanometers, are dispersed evenly throughout a continuous medium without settling. Emulsions are a specific type of colloid involving the dispersion of two immiscible liquids, such as oil and water, stabilized by emulsifying agents. Understanding the particle size, phase distribution, and stabilization mechanisms is crucial for differentiating colloids from emulsions in various industrial and scientific applications.
Defining Colloids: Characteristics and Types
Colloids are mixtures where microscopic particles, ranging from 1 to 1000 nanometers, are dispersed evenly throughout another substance, remaining stable without settling. These systems exhibit unique properties such as the Tyndall effect, and can be classified into types including sols, gels, foams, and emulsions based on the dispersed phase and dispersion medium. Understanding the size, interaction, and phase of particles in colloids is essential for differentiating them from emulsions and other mixtures.
What is an Emulsion? Structure and Properties
An emulsion is a heterogeneous mixture of two immiscible liquids, where one liquid is dispersed as fine droplets within the other, stabilized by emulsifying agents such as surfactants. The structure of an emulsion consists of dispersed droplets ranging from 0.1 to 100 micrometers suspended in a continuous phase, creating either oil-in-water (O/W) or water-in-oil (W/O) systems. Emulsions exhibit distinct properties including instability to coalescence, turbidity, and sensitivity to temperature and pH changes, often requiring stabilizers to maintain their uniform dispersion and prevent separation.
Key Differences Between Colloids and Emulsions
Colloids are heterogeneous mixtures where particles range from 1 to 1000 nanometers, dispersed evenly throughout a continuous medium, while emulsions specifically involve the dispersion of one liquid into another immiscible liquid, typically oil and water. The particle size in emulsions is generally larger than in colloids, leading to visible light scattering and instability without emulsifiers. Colloids exhibit phenomena such as the Tyndall effect and Brownian motion, whereas emulsions rely on surfactants for stability and typically separate over time without stabilizing agents.
Types of Colloids: Classification and Examples
Colloids are classified based on the dispersed phase and dispersing medium, including types such as sols (solid particles in liquid), gels (solid network in liquid), emulsions (liquid droplets in another immiscible liquid), foams (gas in liquid or solid), and aerosols (solid or liquid particles in gas). Emulsions, a subset of colloids, consist specifically of liquid droplets dispersed in another liquid, with common examples like milk (fat in water) and mayonnaise (oil in water). Understanding the classification of colloids by phase state and interaction helps distinguish emulsions and optimize their stability and applications in industries like food, pharmaceuticals, and cosmetics.
Types of Emulsions: Oil-in-Water vs Water-in-Oil
Emulsions are a specific type of colloid composed of two immiscible liquids where one is dispersed in the other as droplets, categorized mainly into oil-in-water (O/W) and water-in-oil (W/O) types. In oil-in-water emulsions, oil droplets are dispersed in a continuous water phase, commonly found in products like milk and salad dressings, whereas water-in-oil emulsions consist of water droplets dispersed within oil, typical in butter and margarine. The stability and application of these emulsions depend on factors like droplet size, emulsifier type, and the ratio of oil to water.
Applications of Colloids in Industry and Daily Life
Colloids find extensive applications in industries such as pharmaceuticals, food production, and cosmetics due to their unique particle size and stability, enabling controlled drug delivery, improved texture in food products, and enhanced formulation of skincare items. In daily life, colloidal systems appear in milk, fog, and paints, providing consistent dispersion and stability crucial for product performance and user experience. Emulsions, a specific type of colloid, are vital in cream-based cosmetics, salad dressings, and lubricants, where the mixing of immiscible liquids enhances functionality and consumer appeal.
Uses of Emulsions in Food and Pharmaceuticals
Emulsions play a crucial role in food products such as mayonnaise, salad dressings, and dairy items by improving texture, flavor, and shelf life through the stable dispersion of oil and water phases. In pharmaceuticals, emulsions facilitate controlled drug delivery, enhance bioavailability, and enable the incorporation of hydrophobic drugs in topical creams, vaccines, and intravenous formulations. The ability of emulsions to stabilize active ingredients and improve formulation aesthetics makes them indispensable in both food technology and pharmaceutical applications.
Stability and Separation of Colloids and Emulsions
Colloids exhibit high stability due to the small particle size (1-1000 nm) and Brownian motion, which prevents sedimentation or creaming, whereas emulsions tend to separate over time because larger droplets (typically >1000 nm) coalesce and phase separation occurs. Colloidal stability is maintained by electrostatic or steric stabilization mechanisms, while emulsions require emulsifiers like surfactants to reduce interfacial tension and enhance dispersion stability. Separation in emulsions often results in creaming, sedimentation, or coalescence, leading to phase inversion or breakdown if stabilizers are inadequate.
Summary: Choosing Between Colloid and Emulsion
Colloids contain particles ranging from 1 to 1000 nanometers that remain evenly dispersed without settling, ideal for applications requiring stable mixtures like paints and gels. Emulsions consist of liquid droplets dispersed in another liquid, typically requiring emulsifiers for stability, commonly found in food products and cosmetics. Selecting between colloid and emulsion depends on particle size, phase states, and the need for stability under specific conditions.
Colloid Infographic
 libterm.com
libterm.com