Nitrate aerosols significantly influence air quality and climate by affecting atmospheric chemistry and cloud formation. Their sources include agricultural emissions, industrial activities, and vehicle exhaust, contributing to particulate pollution and health risks. Discover how nitrate aerosols impact your environment and ways to mitigate their effects in the rest of this article.
Table of Comparison
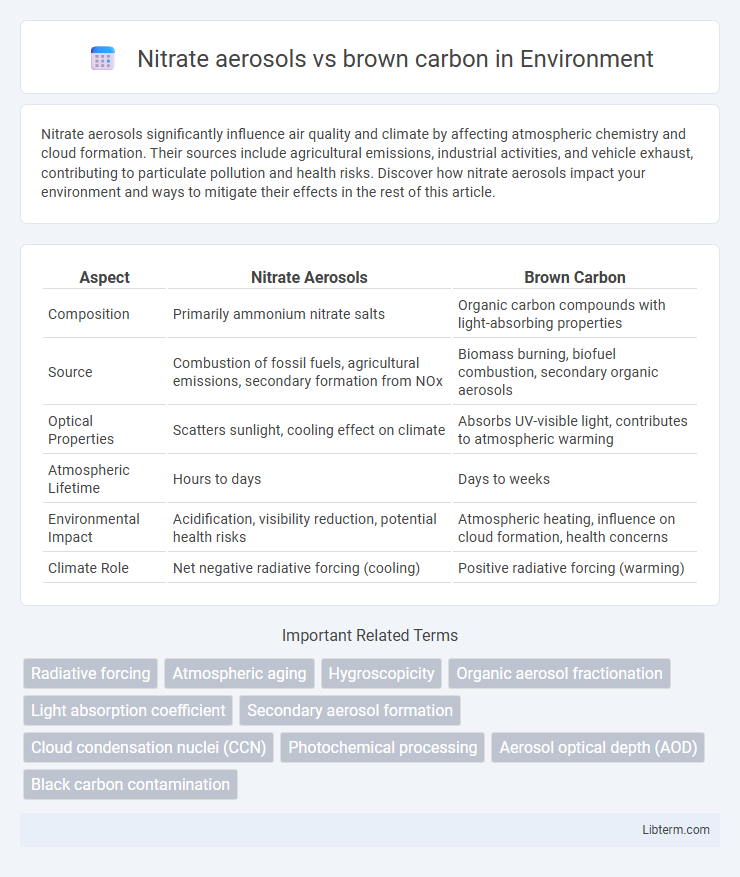
| Aspect | Nitrate Aerosols | Brown Carbon |
|---|---|---|
| Composition | Primarily ammonium nitrate salts | Organic carbon compounds with light-absorbing properties |
| Source | Combustion of fossil fuels, agricultural emissions, secondary formation from NOx | Biomass burning, biofuel combustion, secondary organic aerosols |
| Optical Properties | Scatters sunlight, cooling effect on climate | Absorbs UV-visible light, contributes to atmospheric warming |
| Atmospheric Lifetime | Hours to days | Days to weeks |
| Environmental Impact | Acidification, visibility reduction, potential health risks | Atmospheric heating, influence on cloud formation, health concerns |
| Climate Role | Net negative radiative forcing (cooling) | Positive radiative forcing (warming) |
Introduction to Nitrate Aerosols and Brown Carbon
Nitrate aerosols are fine particulate matter composed primarily of ammonium nitrate formed through atmospheric reactions involving nitrogen oxides and ammonia, significantly impacting air quality and climate by influencing radiative forcing and cloud formation. Brown carbon consists of light-absorbing organic carbon compounds produced by biomass burning and atmospheric chemical reactions, contributing to atmospheric warming by absorbing solar radiation in the ultraviolet and visible spectrum. Both nitrate aerosols and brown carbon play critical roles in atmospheric chemistry, affecting visibility, human health, and climate processes through distinct optical and chemical properties.
Chemical Composition and Sources
Nitrate aerosols primarily consist of ammonium nitrate formed from the reaction of ammonia with nitric acid, originating mainly from agricultural activities and vehicular emissions. Brown carbon comprises complex organic molecules rich in light-absorbing functional groups such as humic-like substances, emitted predominantly by biomass burning and combustion of fossil fuels. The distinct chemical compositions influence their environmental roles, with nitrate aerosols affecting particulate matter acidity and brown carbon significantly contributing to atmospheric light absorption and climate forcing.
Formation Mechanisms
Nitrate aerosols primarily form through the reaction of nitrogen oxides (NOx) with ammonia (NH3) or other alkaline substances in the atmosphere, resulting in ammonium nitrate via gas-to-particle conversion. Brown carbon arises from the incomplete combustion of organic matter and secondary organic aerosol formation, involving complex photochemical reactions of volatile organic compounds (VOCs) that generate light-absorbing organic compounds. Both nitrate aerosols and brown carbon significantly influence atmospheric chemistry and climate by altering aerosol optical properties and serving as cloud condensation nuclei.
Atmospheric Distribution and Lifespan
Nitrate aerosols predominantly form in polluted urban and agricultural areas with high ammonia and nitrogen oxide emissions, exhibiting a seasonal distribution peaking in colder months due to enhanced secondary formation. Brown carbon, primarily emitted from biomass burning and fossil fuel combustion, shows a broader spatial distribution influenced by wildfire events and anthropogenic activities, with more extended atmospheric lifespans in the upper troposphere due to slower photochemical aging. The atmospheric lifetime of nitrate aerosols ranges from hours to days, influenced by humidity and temperature, whereas brown carbon can persist for several days to weeks depending on atmospheric conditions and chemical transformations.
Optical Properties and Light Absorption
Nitrate aerosols exhibit weak light absorption with strong scattering primarily in the ultraviolet and visible wavelengths, contributing to atmospheric cooling by reflecting sunlight. Brown carbon, a component of organic aerosols, shows significant absorption in the ultraviolet and short visible spectrum due to its chromophoric organic compounds, enhancing atmospheric warming effects. The contrasting optical properties result in nitrate aerosols reducing radiative forcing, while brown carbon increases it by absorbing solar radiation and influencing cloud formation.
Climate Impacts and Radiative Forcing
Nitrate aerosols significantly contribute to atmospheric cooling by scattering solar radiation and increasing cloud albedo, leading to negative radiative forcing effects. Brown carbon absorbs ultraviolet and visible light, enhancing atmospheric warming through positive radiative forcing and altering regional climate patterns. The contrasting radiative properties of nitrate aerosols and brown carbon influence climate models by affecting the Earth's energy balance and cloud formation processes.
Influence on Air Quality and Human Health
Nitrate aerosols significantly contribute to fine particulate matter (PM2.5), leading to respiratory issues and cardiovascular diseases due to their deep lung penetration and inflammatory effects. Brown carbon, a component of organic aerosols, absorbs light and exacerbates air pollution by increasing atmospheric warming and oxidative stress, which can impair lung function and trigger asthma. Both aerosols degrade air quality by enhancing atmospheric oxidative capacity and promoting secondary pollutant formation, thus posing substantial risks to human health.
Role in Cloud Formation and Precipitation
Nitrate aerosols serve as efficient cloud condensation nuclei (CCN), enhancing cloud droplet formation and influencing cloud albedo and lifetime, which can modulate local and regional precipitation patterns. Brown carbon, composed of light-absorbing organic compounds, impacts cloud microphysics by altering atmospheric radiation balance and affecting ice nucleation processes, thus modifying cloud formation and potentially suppressing precipitation. The distinct physicochemical properties of nitrate aerosols and brown carbon drive their contrasting roles in cloud dynamics and precipitation regulation, with nitrate aerosols promoting cloud droplet nucleation and brown carbon influencing radiative forcing and cloud phase transition.
Regional and Seasonal Variability
Nitrate aerosols exhibit pronounced regional variability, with elevated concentrations in industrial and agricultural areas, especially during colder seasons due to enhanced nitrate formation under low temperatures. Brown carbon shows significant seasonal fluctuations, intensifying during biomass burning periods in dry seasons and influencing radiative forcing over tropical and subtropical regions. Both aerosol types impact air quality and climate differently across regions, with nitrate aerosols contributing to secondary inorganic particulate matter and brown carbon primarily originating from incomplete combustion, modulating atmospheric absorption characteristics significantly.
Future Research and Mitigation Strategies
Future research on nitrate aerosols and brown carbon should prioritize their differential impacts on climate forcing and air quality through advanced atmospheric modeling and field measurements. Understanding the sources, chemical transformations, and deposition patterns of these aerosols will improve the accuracy of climate predictions and health risk assessments. Mitigation strategies must integrate emission controls targeting nitrogen oxides and organic carbon precursors, alongside innovations in renewable energy and urban planning to reduce overall aerosol burden effectively.
Nitrate aerosols Infographic
 libterm.com
libterm.com