The oxide layer forms naturally on metal surfaces, providing a protective barrier against corrosion and environmental damage. Its thickness and composition vary depending on the metal type and exposure conditions, significantly influencing the material's durability and performance. Explore the rest of this article to understand how the oxide layer affects your metal products and ways to enhance its protective properties.
Table of Comparison
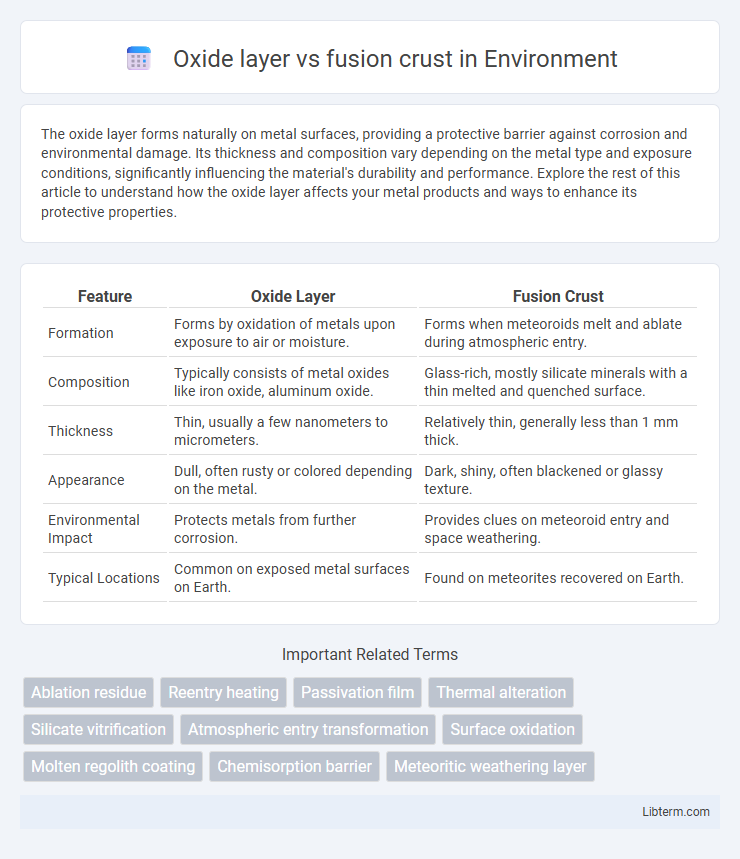
| Feature | Oxide Layer | Fusion Crust |
|---|---|---|
| Formation | Forms by oxidation of metals upon exposure to air or moisture. | Forms when meteoroids melt and ablate during atmospheric entry. |
| Composition | Typically consists of metal oxides like iron oxide, aluminum oxide. | Glass-rich, mostly silicate minerals with a thin melted and quenched surface. |
| Thickness | Thin, usually a few nanometers to micrometers. | Relatively thin, generally less than 1 mm thick. |
| Appearance | Dull, often rusty or colored depending on the metal. | Dark, shiny, often blackened or glassy texture. |
| Environmental Impact | Protects metals from further corrosion. | Provides clues on meteoroid entry and space weathering. |
| Typical Locations | Common on exposed metal surfaces on Earth. | Found on meteorites recovered on Earth. |
Introduction to Meteorite Surfaces
Meteorite surfaces display distinct features such as oxide layers and fusion crusts, which provide valuable insights into their atmospheric entry and composition. Oxide layers form through chemical reactions between the meteorite's minerals and oxygen, indicating prolonged exposure to Earth's environment. Fusion crusts result from intense heat during atmospheric entry, creating a glassy outer shell that protects the meteorite's interior from further alteration.
Defining Oxide Layer and Fusion Crust
The oxide layer is a thin film of metal oxide that forms on the surface of a metal when it reacts with oxygen, often resulting in corrosion protection or altered surface properties. Fusion crust is the dark, glassy outer shell on a meteorite created by intense heat and melting as it passes through Earth's atmosphere. Both layers represent surface alterations but differ fundamentally in formation processes--chemical oxidation versus thermal melting.
Formation Process of Oxide Layer
The oxide layer forms through a chemical reaction when metals are exposed to oxygen, typically occurring at elevated temperatures that facilitate oxidation. This process results in a thin, protective film of metal oxides on the surface, which slows further corrosion. Fusion crust, by contrast, develops from the rapid melting and cooling of a meteorite's surface during atmospheric entry, creating a glassy, often blackened outer layer.
How Fusion Crust Develops
Fusion crust develops when a meteoroid enters Earth's atmosphere at high velocity, causing the outer layer to rapidly heat and partially melt due to intense frictional forces. This molten surface quickly cools and solidifies upon deceleration, forming a thin, glassy, and dark crust composed mainly of iron oxides and silicate glass. In contrast, the oxide layer forms more slowly through long-term exposure to Earth's atmosphere, resulting in a uniform, often thicker, rust-like coating primarily composed of iron oxides.
Chemical Composition Comparison
Oxide layers primarily form through the oxidation of metal surfaces, resulting in compounds such as iron oxide (Fe2O3) or aluminum oxide (Al2O3), which exhibit a relatively uniform and stable chemical composition. Fusion crusts develop when meteoroids enter Earth's atmosphere, melting and rapidly cooling their exterior, creating a chemically heterogeneous layer with a mixture of silicates, oxides, and sometimes metal inclusions. The fusion crust often contains higher concentrations of volatile elements and altered mineral phases compared to the more chemically consistent oxide layer found on terrestrial metals.
Structural Differences
The oxide layer forms as a thin, uniform coating of metal oxides on the surface of materials exposed to oxidizing environments, characterized by its crystalline structure and adherence to the base material. Fusion crust appears on meteorites as a glassy, irregularly textured outer layer created by melting and rapid cooling during atmospheric entry, representing a distinct, non-crystalline cooling phase. Structural differences include the oxide layer's stable, crystalline lattice versus the fusion crust's amorphous, brittle glass composition formed from high-temperature ablation.
Visual and Textural Features
The oxide layer on metals typically presents a smooth, uniform surface with a dull or slightly glossy finish due to the controlled oxidation process, characterized by thin, consistent thickness and often exhibiting color variations like blue or brown hues. The fusion crust on meteorites, formed by rapid melting and cooling during atmospheric entry, displays a dark, glassy, and irregularly cracked texture with a matte appearance, often interspersed with vesicles or flow patterns. Texturally, oxide layers are more homogeneous and adherent, while fusion crusts are brittle, uneven, and sometimes flake off due to thermal shock.
Role in Meteorite Identification
The oxide layer and fusion crust serve distinct roles in meteorite identification, with the fusion crust providing a thin, glassy outer shell formed from melting during atmospheric entry, crucial for confirming recent terrestrial arrival. The oxide layer, often forming beneath or adjacent to the fusion crust through weathering processes, helps determine the meteorite's terrestrial age and alteration history. Analyzing both features together enhances accurate classification and differentiation from terrestrial rocks and weathered meteorites.
Preservation and Weathering Effects
The oxide layer, formed on metal surfaces through oxidation, often preserves the underlying material but is susceptible to gradual weathering, leading to corrosion and structural weakening. Fusion crust, a glassy outer coating on meteorites created during atmospheric entry, acts as a protective barrier that slows weathering but eventually fractures and weathers due to environmental exposure. Both layers serve as initial preservation shields but differ in composition and durability under terrestrial weathering conditions.
Scientific Importance of Each Layer
The oxide layer, formed through the oxidation of metals, plays a critical role in protecting materials from corrosion and enhancing durability in various industrial applications. The fusion crust, a thin glassy coating created when meteoroids melt upon entering Earth's atmosphere, provides valuable scientific insight into the thermal and chemical processes during atmospheric entry. Understanding both layers is essential for advancements in materials science and planetary geology, enabling improvements in corrosion resistance and the study of extraterrestrial objects.
Oxide layer Infographic
 libterm.com
libterm.com