Acidic soil has a pH level below 7, which can limit nutrient availability and affect plant growth. Understanding how to manage and amend acidic soil is essential for improving your garden's health and productivity. Discover effective strategies to balance soil pH and enhance your plants' vitality in the full article.
Table of Comparison
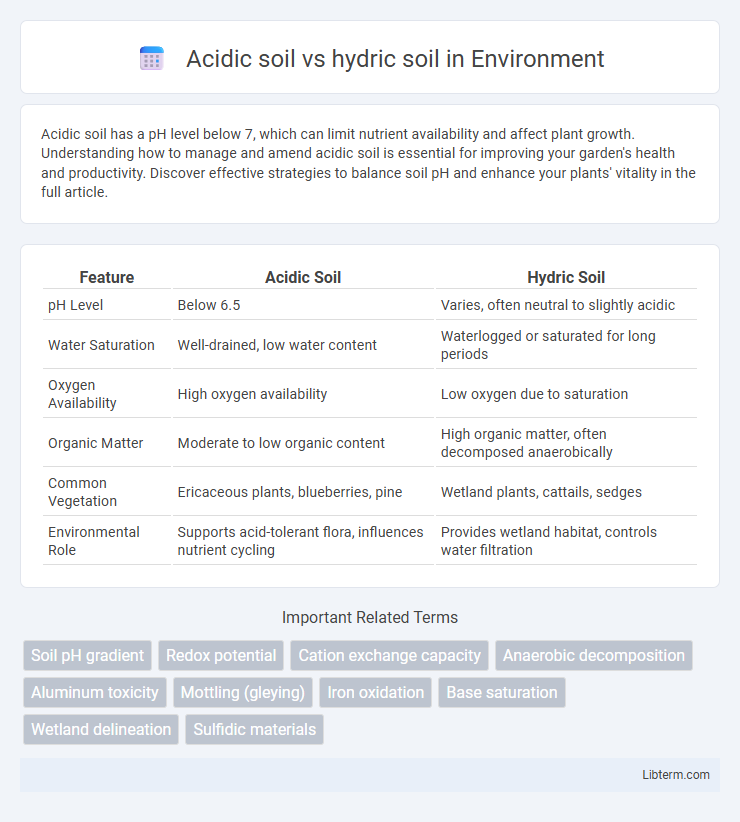
| Feature | Acidic Soil | Hydric Soil |
|---|---|---|
| pH Level | Below 6.5 | Varies, often neutral to slightly acidic |
| Water Saturation | Well-drained, low water content | Waterlogged or saturated for long periods |
| Oxygen Availability | High oxygen availability | Low oxygen due to saturation |
| Organic Matter | Moderate to low organic content | High organic matter, often decomposed anaerobically |
| Common Vegetation | Ericaceous plants, blueberries, pine | Wetland plants, cattails, sedges |
| Environmental Role | Supports acid-tolerant flora, influences nutrient cycling | Provides wetland habitat, controls water filtration |
Understanding Acidic Soil: Definition and Characteristics
Acidic soil is defined by a pH level below 7, often ranging between 4 and 6, which significantly influences nutrient availability and microbial activity. It typically contains high concentrations of hydrogen ions and may be rich in aluminum and iron compounds, affecting plant growth. Characteristics include poor drainage, low base saturation, and a tendency to support acid-loving vegetation such as blueberries and azaleas.
What Is Hydric Soil? Key Features Explained
Hydric soil is characterized by saturation, flooding, or ponding long enough during the growing season to develop anaerobic conditions, critical for supporting wetland vegetation. Unlike acidic soil, which has a low pH due to high hydrogen ion concentration affecting nutrient availability, hydric soil supports unique microbial processes and organic matter accumulation in anaerobic environments. Key features of hydric soil include gleyed colors, high organic content, and redoximorphic features indicating periodic saturation and reduction-oxidation reactions.
Formation Processes: How Acidic Soils and Hydric Soils Develop
Acidic soils develop primarily through the leaching of basic cations like calcium and magnesium, often in regions with high rainfall that promote organic matter decomposition and acidification. Hydric soils form under anaerobic conditions in saturated environments such as wetlands, where slow decomposition rates and waterlogging result in the accumulation of organic materials and reduced iron compounds. Both soil types reflect distinct environmental formation processes that influence their chemical and physical properties.
pH Levels: Acidic vs Hydric Soil Composition
Acidic soil typically has a pH below 7, often ranging between 4 and 6, due to high concentrations of hydrogen ions and organic acids resulting from decomposition processes. Hydric soil, characterized by prolonged saturation, usually exhibits reduced oxygen levels, which affects redox reactions and can cause pH to vary widely, often tending toward neutral or slightly acidic conditions influenced by anaerobic microbial activity. The distinct pH profiles in acidic versus hydric soils directly impact nutrient availability, microbial communities, and plant root development.
Nutrient Availability in Acidic and Hydric Soils
Nutrient availability in acidic soils is typically low due to the increased solubility of toxic metals like aluminum and iron, which interfere with plant nutrient uptake and reduce essential elements such as calcium, magnesium, and phosphorus. Hydric soils, often saturated or flooded, exhibit reduced oxygen levels that limit microbial activity, thereby affecting nitrogen cycling and decreasing the availability of nitrates for plants. Both soil types present unique challenges for nutrient management, requiring specific amendments and cultivation practices to optimize plant growth.
Impact on Plant Growth: Comparing Acidic and Hydric Environments
Acidic soils, characterized by a low pH below 5.5, often limit nutrient availability such as calcium, magnesium, and phosphorus, which can inhibit plant growth and reduce crop yields. Hydric soils are saturated with water, creating anaerobic conditions that affect root respiration and promote the accumulation of toxic elements like iron and manganese, hindering oxygen uptake. Plants adapted to acidic soils typically have mechanisms to tolerate aluminum toxicity, whereas those in hydric environments possess aerenchyma tissues to facilitate oxygen transport in waterlogged conditions.
Common Vegetation: Plant Species in Acidic vs Hydric Soils
Acidic soils commonly support vegetation such as blueberries (Vaccinium spp.), rhododendrons (Rhododendron spp.), and pitch pines (Pinus rigida), which thrive in low pH and nutrient-poor conditions. Hydric soils foster wetland plants like cattails (Typha spp.), sedges (Carex spp.), and water lilies (Nymphaea spp.) adapted to saturated, oxygen-poor environments. The distinct plant species reflect adaptations to pH levels and soil moisture, influencing ecosystem biodiversity and function.
Soil Management Techniques for Acidic and Hydric Conditions
Soil management techniques for acidic soils involve liming to raise pH levels and improve nutrient availability, alongside organic matter additions to enhance soil structure and microbial activity. Hydric soil management focuses on controlling water saturation through drainage systems and selecting flood-tolerant plant species to prevent anaerobic conditions and promote healthy root development. Both soil types benefit from tailored nutrient management plans and monitoring to optimize soil health and crop productivity.
Environmental Significance: Ecosystem Roles of Each Soil Type
Acidic soils with pH levels below 5.5 support unique plant communities such as blueberries and conifers, influencing carbon sequestration and nutrient cycling in forest ecosystems. Hydric soils, characterized by saturation and anaerobic conditions, create wetlands that provide crucial habitats for diverse aquatic species and act as natural buffers for flood control and water purification. Both soil types play essential roles in sustaining biodiversity and regulating ecosystem services within their respective environments.
Choosing the Right Soil for Agriculture and Landscaping
Acidic soil contains a low pH, often below 7, and is rich in hydrogen ions, affecting nutrient availability for crops and plants, which is crucial when selecting soil for agriculture and landscaping. Hydric soil, saturated or flooded long enough to develop anaerobic conditions, supports wetland vegetation and contributes to water filtration but may limit crop types due to oxygen deprivation in roots. Choosing the right soil depends on crop tolerance to pH and drainage conditions, with acidic soils favoring acidophilic plants and hydric soils best suited for wetlands, riparian buffers, or landscape areas requiring water retention.
Acidic soil Infographic
 libterm.com
libterm.com