Alpha-pinene is a natural organic compound classified as a monoterpene, commonly found in pine trees and other conifers, known for its distinctive pine aroma and potential therapeutic properties. Research highlights its anti-inflammatory, antimicrobial, and bronchodilator effects, making it a valuable component in aromatherapy and natural health products. Discover how alpha-pinene can benefit your wellness and explore detailed insights in the rest of the article.
Table of Comparison
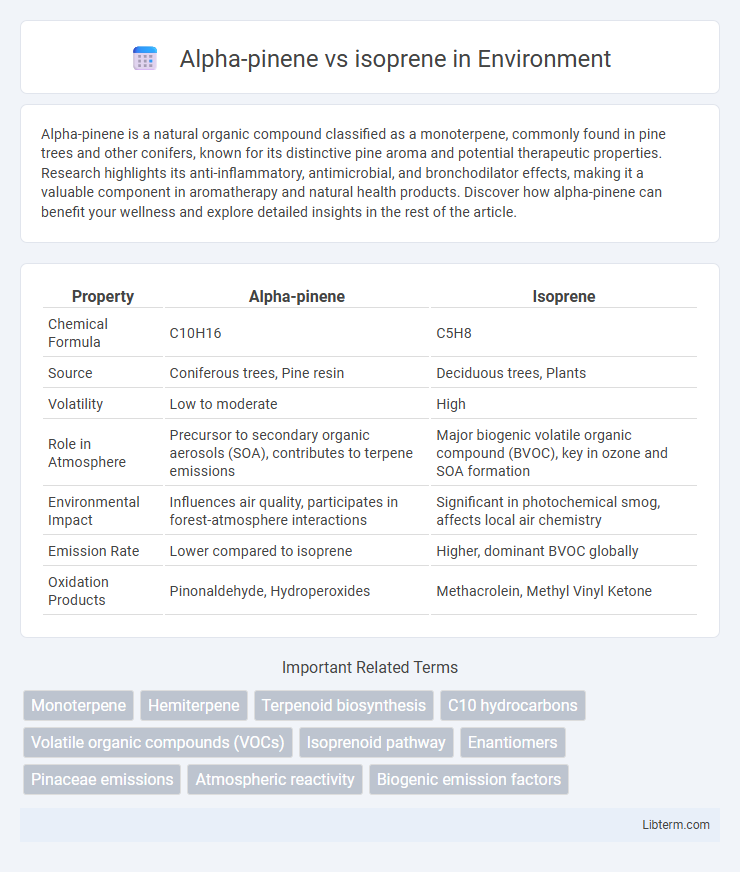
| Property | Alpha-pinene | Isoprene |
|---|---|---|
| Chemical Formula | C10H16 | C5H8 |
| Source | Coniferous trees, Pine resin | Deciduous trees, Plants |
| Volatility | Low to moderate | High |
| Role in Atmosphere | Precursor to secondary organic aerosols (SOA), contributes to terpene emissions | Major biogenic volatile organic compound (BVOC), key in ozone and SOA formation |
| Environmental Impact | Influences air quality, participates in forest-atmosphere interactions | Significant in photochemical smog, affects local air chemistry |
| Emission Rate | Lower compared to isoprene | Higher, dominant BVOC globally |
| Oxidation Products | Pinonaldehyde, Hydroperoxides | Methacrolein, Methyl Vinyl Ketone |
Introduction to Alpha-Pinene and Isoprene
Alpha-pinene is a bicyclic monoterpene commonly found in the essential oils of pine trees and conifers, characterized by its distinctive pine-like aroma and significant role in atmospheric chemistry. Isoprene, a volatile hydrocarbon emitted predominantly by many plants and trees, serves as a fundamental building block for natural rubber and plays a crucial role in forming atmospheric aerosols. Both compounds contribute to biogenic VOC emissions, influencing air quality and climate through their reactive chemical properties.
Chemical Structure Comparison
Alpha-pinene features a bicyclic monoterpene structure with a rigid framework composed of two fused rings, characterized by a double bond within one ring, while isoprene is a simple acyclic diene consisting of a five-carbon backbone with two conjugated double bonds. The molecular formula for alpha-pinene is C10H16, reflecting its monoterpene classification, whereas isoprene, C5H8, serves as the fundamental building block for many terpenes and natural rubber. Differences in ring strain and conjugation between alpha-pinene and isoprene significantly influence their chemical reactivity and biosynthetic pathways.
Natural Sources and Occurrence
Alpha-pinene is predominantly found in coniferous trees such as pines and spruces, representing a major component of turpentine and contributing to forest aromas. Isoprene originates mainly from the emission of deciduous trees, especially oaks and eucalyptus, constituting the largest fraction of biogenic volatile organic compounds released into the atmosphere. Both compounds play crucial roles in atmospheric chemistry but differ significantly in their plant sources and environmental distributions.
Biosynthetic Pathways
Alpha-pinene is synthesized through the mevalonate pathway, where geranyl pyrophosphate (GPP) serves as a key intermediate in monoterpene biosynthesis. In contrast, isoprene is produced via the methylerythritol phosphate (MEP) pathway in plastids, originating from pyruvate and glyceraldehyde-3-phosphate. Both compounds derive from isopentenyl pyrophosphate (IPP) but diverge early in their biosynthetic routes, leading to distinct enzymatic reactions and structural outcomes.
Physical and Chemical Properties
Alpha-pinene is a bicyclic monoterpene with a molecular formula of C10H16, exhibiting a boiling point around 155degC and a density of 0.858 g/cm3, characterized by its pine-like aroma and insolubility in water. Isoprene, a volatile diene hydrocarbon with formula C5H8, has a lower boiling point near 34degC and density approximately 0.681 g/cm3, notable for its high reactivity due to conjugated double bonds enabling polymerization. Chemical stability differs as alpha-pinene undergoes oxidation to form compounds such as verbenone, while isoprene readily participates in copolymer formation, influencing its industrial application in synthetic rubber production.
Industrial and Commercial Applications
Alpha-pinene, a major component of turpentine oil, is extensively used in the production of synthetic fragrances, resins, and pharmaceuticals due to its pleasant pine aroma and chemical reactivity. Isoprene serves as a fundamental building block in the synthetic rubber industry, particularly in manufacturing polyisoprene and butyl rubber, essential for automotive tires and adhesives. Both compounds hold significant commercial value, with alpha-pinene driving applications in fine chemicals and isoprene dominating elastomer production.
Environmental Impact and Emissions
Alpha-pinene, a monoterpene emitted primarily by coniferous trees, contributes to the formation of secondary organic aerosols that can influence air quality and climate by affecting cloud condensation nuclei. Isoprene, emitted in larger quantities by deciduous trees and grasses, plays a significant role in tropospheric ozone formation and can exacerbate urban smog, impacting human health and vegetation. Both compounds act as biogenic volatile organic compounds (BVOCs), but isoprene's higher reactivity leads to greater contributions to ground-level ozone and photochemical smog compared to alpha-pinene, influencing regional air pollution models and mitigation strategies.
Health Effects and Safety Profiles
Alpha-pinene, a terpene found in coniferous trees, exhibits anti-inflammatory and bronchodilator properties, making it beneficial for respiratory health, but high exposure may cause irritation or allergic reactions. Isoprene, a volatile hydrocarbon primarily emitted by plants, poses greater risks due to its potential to form harmful secondary pollutants and respiratory irritants upon oxidation, leading to concerns about long-term exposure effects. Both compounds require careful handling in industrial and environmental settings to minimize health risks and ensure safety compliance.
Role in Plant Biology and Ecology
Alpha-pinene, a monoterpene prevalent in coniferous trees, plays a crucial role in plant defense mechanisms by deterring herbivores and attracting predatory insects, while also contributing to atmospheric chemical processes that influence plant communication. Isoprene, the most abundantly emitted volatile hydrocarbon from many plant species, enhances thermal tolerance and stabilizes cellular membranes under stress conditions, thus supporting photosynthetic efficiency and overall plant resilience. Both compounds significantly impact ecological interactions by modulating plant-environment dynamics and influencing atmospheric chemistry related to plant volatile organic compounds (VOCs).
Future Research and Innovations
Future research on Alpha-pinene and isoprene targets the exploration of their biosynthetic pathways to enhance sustainable production methods for biofuels and bio-based chemicals. Innovations in metabolic engineering aim to optimize microbial synthesis of Alpha-pinene for high-value pharmaceuticals and isoprene for elastomer manufacturing. Advanced analytical techniques and computational modeling drive the identification of novel derivatives with improved industrial applications and environmental benefits.
Alpha-pinene Infographic
 libterm.com
libterm.com