Isopentane, also known as 2-methylbutane, is a highly flammable hydrocarbon used primarily as a blowing agent in the production of polystyrene foam and as a solvent in laboratories. Its low boiling point and excellent volatility make it valuable in refrigeration and fuel applications. Discover more about isopentane's properties, uses, and safety considerations in the rest of this article.
Table of Comparison
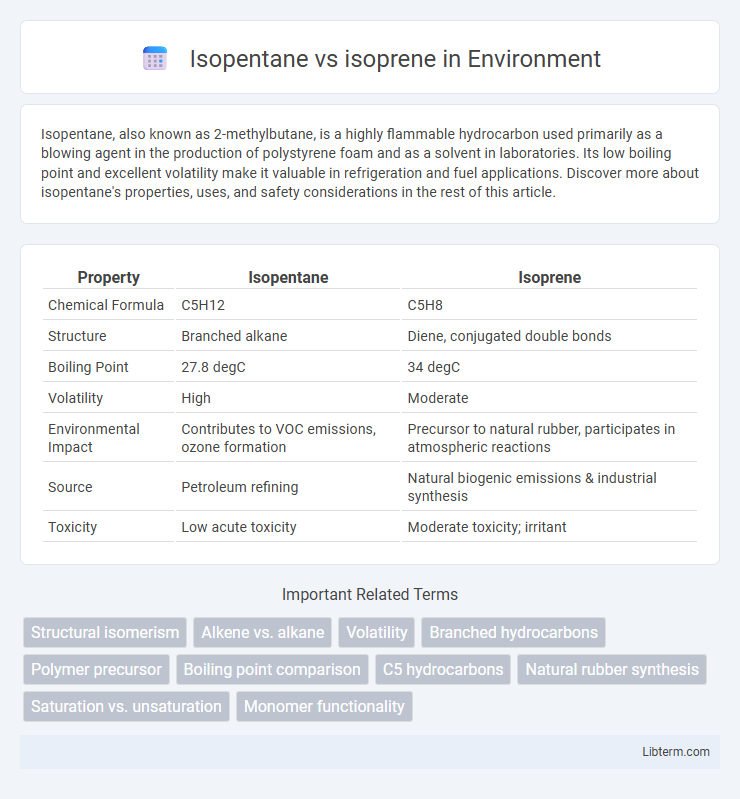
| Property | Isopentane | Isoprene |
|---|---|---|
| Chemical Formula | C5H12 | C5H8 |
| Structure | Branched alkane | Diene, conjugated double bonds |
| Boiling Point | 27.8 degC | 34 degC |
| Volatility | High | Moderate |
| Environmental Impact | Contributes to VOC emissions, ozone formation | Precursor to natural rubber, participates in atmospheric reactions |
| Source | Petroleum refining | Natural biogenic emissions & industrial synthesis |
| Toxicity | Low acute toxicity | Moderate toxicity; irritant |
Introduction to Isopentane and Isoprene
Isopentane (C5H12) is a branched alkane used primarily as a solvent and in the production of blowing agents for foam insulation, characterized by its saturated hydrocarbon structure with five carbon atoms. Isoprene (C5H8), on the other hand, is an unsaturated hydrocarbon and a key monomer in the biosynthesis of natural rubber, notable for its conjugated diene system that enables polymerization. Both compounds share the same carbon count but differ significantly in chemical structure and industrial applications, with isopentane being a stable alkane and isoprene serving as a reactive building block in polymer chemistry.
Chemical Structures and Formulas
Isopentane (C5H12) is a branched alkane with a saturated hydrocarbon structure featuring only single bonds, consisting of a central carbon atom bonded to three methyl groups and one ethyl group. In contrast, isoprene (C5H8) is a diene hydrocarbon containing two conjugated double bonds within its five-carbon chain, making it unsaturated and highly reactive in polymerization processes. The structural difference between the saturated isopentane and the unsaturated isoprene significantly influences their chemical properties and applications.
Sources and Production Methods
Isopentane is primarily derived from natural gas liquids and petroleum refining processes, with steam cracking of hydrocarbons being a common production method. In contrast, isoprene is mostly obtained through the thermal cracking of naphtha or by biotechnological fermentation using engineered microorganisms that convert sugars into isoprene. While isopentane production relies heavily on fossil fuel feedstocks, isoprene production increasingly explores sustainable bio-based routes to meet industrial demand.
Physical Properties Comparison
Isopentane, a branched alkane with the formula C5H12, has a boiling point of about 27.8degC and a melting point near -160degC, reflecting its nonpolar, saturated hydrocarbon structure. Isoprene, a diene with the formula C5H8, exhibits a slightly higher boiling point around 34degC and a melting point near -145degC due to its unsaturated double bonds and conjugated system. The differences in molecular structure contribute to variations in volatility, solubility, and reactivity, with isoprene being more chemically reactive and slightly less volatile than isopentane under standard conditions.
Common Industrial Applications
Isopentane is widely used as a blowing agent in the production of polystyrene foam insulation and as a solvent in the pharmaceutical industry, while isoprene serves primarily as a key monomer in the manufacture of synthetic rubber, including polyisoprene for tires and adhesives. Isopentane's volatility and low boiling point make it ideal for refrigeration and fuel applications, whereas isoprene's reactive diene structure supports polymerization processes crucial for elastomer production. Both hydrocarbons are essential in industrial chemistry but target distinct markets: isopentane in foam and solvent applications, and isoprene in rubber and elastomer manufacturing.
Safety and Handling Considerations
Isopentane is a highly flammable liquid with a low flash point, requiring storage in well-ventilated areas away from ignition sources and use of grounded containers to prevent static discharge. Isoprene, also highly flammable and volatile, poses additional hazards due to its tendency to polymerize spontaneously, necessitating storage with inhibitors and temperature control to prevent explosive polymerization. Both compounds require the use of appropriate personal protective equipment (PPE), including gloves and eye protection, and adherence to strict handling protocols to minimize inhalation and skin contact risks.
Environmental Impact and Biodegradability
Isopentane is a branched alkane with limited biodegradability and moderate environmental persistence, commonly used as a blowing agent and solvent, posing risks of air pollution and groundwater contamination if released extensively. Isoprene, a natural hydrocarbon emitted by many plants, significantly contributes to atmospheric reactions influencing ozone formation and secondary organic aerosol production, but it is more readily biodegraded by microorganisms due to its unsaturated structure. The environmental impact of isoprene is nuanced by its role in natural biogeochemical cycles, while isopentane's synthetic origin results in greater concern over long-term ecological accumulation.
Isopentane vs Isoprene: Performance Differences
Isopentane exhibits higher volatility and is commonly used as a blowing agent or solvent, while isoprene serves primarily as a monomer in synthetic rubber production due to its polymerizable double bonds. The performance differences stem from isopentane's saturated hydrocarbon structure leading to lower reactivity, whereas isoprene's conjugated diene structure enables copolymerization, enhancing elasticity in elastomers. Thermal stability also varies, with isopentane vaporizing at 27.8degC and isoprene polymerizing under mild conditions, reflecting their distinct industrial applications and performance characteristics.
Cost and Market Availability
Isopentane, a branched alkane widely used as a blowing agent and solvent, typically has lower market prices due to its simpler production processes and high availability as a petroleum derivative. Isoprene, a key monomer for synthetic rubber production, commands higher costs reflecting its more complex synthesis from petrochemical feedstocks and limited supply compared to isopentane. Market demand for isoprene remains strong in the automotive and tire industries, influencing its price volatility, whereas isopentane's broader industrial applications contribute to stable availability and more competitive pricing.
Conclusion: Choosing Between Isopentane and Isoprene
Selecting between isopentane and isoprene depends on the specific industrial application and chemical properties required; isopentane is favored for its low boiling point and use as a refrigerant or blowing agent, while isoprene is essential in synthetic rubber production due to its polymerizable diene structure. Safety considerations and reactivity also influence the choice, with isopentane exhibiting lower reactivity compared to the more reactive, double-bond containing isoprene. Evaluating cost-effectiveness, environmental impact, and application needs ensures optimal utilization of these hydrocarbons in chemical manufacturing.
Isopentane Infographic
 libterm.com
libterm.com