Polycyclic aromatic hydrocarbons (PAHs) are organic compounds composed of multiple fused aromatic rings that pose significant environmental and health risks. These pollutants originate from incomplete combustion of carbon-containing fuels and are found in air, soil, and water, often linked to cancer and respiratory issues. Discover how understanding PAHs can help you minimize exposure and safeguard your health in the full article.
Table of Comparison
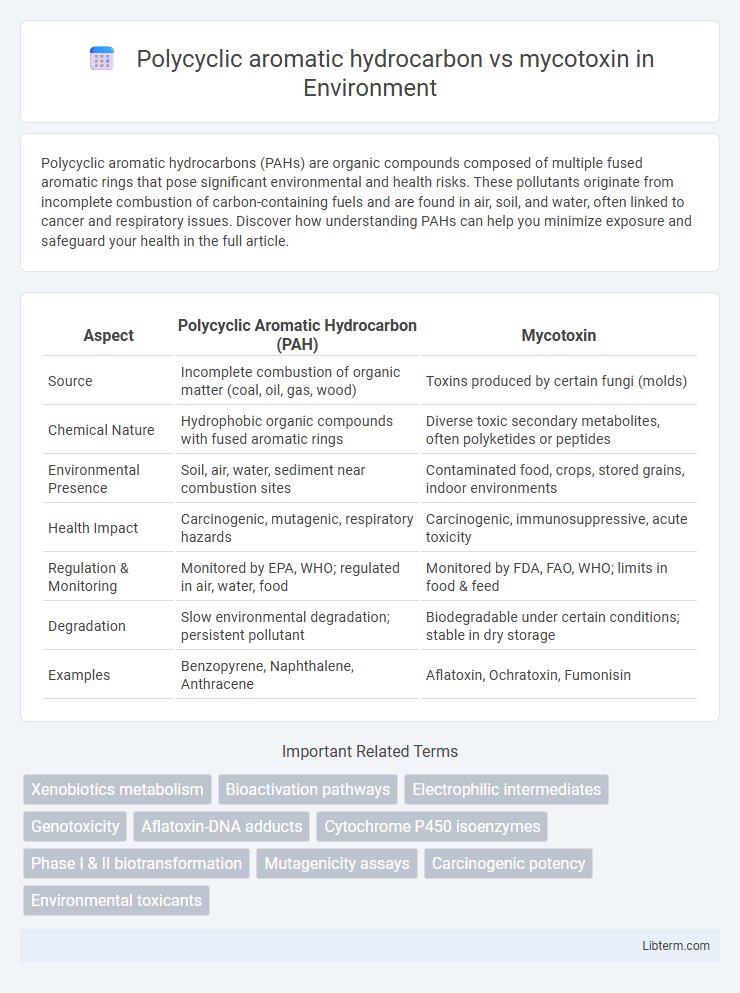
| Aspect | Polycyclic Aromatic Hydrocarbon (PAH) | Mycotoxin |
|---|---|---|
| Source | Incomplete combustion of organic matter (coal, oil, gas, wood) | Toxins produced by certain fungi (molds) |
| Chemical Nature | Hydrophobic organic compounds with fused aromatic rings | Diverse toxic secondary metabolites, often polyketides or peptides |
| Environmental Presence | Soil, air, water, sediment near combustion sites | Contaminated food, crops, stored grains, indoor environments |
| Health Impact | Carcinogenic, mutagenic, respiratory hazards | Carcinogenic, immunosuppressive, acute toxicity |
| Regulation & Monitoring | Monitored by EPA, WHO; regulated in air, water, food | Monitored by FDA, FAO, WHO; limits in food & feed |
| Degradation | Slow environmental degradation; persistent pollutant | Biodegradable under certain conditions; stable in dry storage |
| Examples | Benzopyrene, Naphthalene, Anthracene | Aflatoxin, Ochratoxin, Fumonisin |
Introduction to Polycyclic Aromatic Hydrocarbons and Mycotoxins
Polycyclic aromatic hydrocarbons (PAHs) are a group of organic compounds composed of multiple fused aromatic rings, commonly formed during incomplete combustion of organic matter. Mycotoxins are toxic secondary metabolites produced by certain species of fungi, often contaminating food and feed crops under specific environmental conditions. Both PAHs and mycotoxins pose significant health risks due to their carcinogenic, mutagenic, and immunosuppressive properties, requiring careful monitoring in environmental and food safety contexts.
Chemical Structure: PAHs vs. Mycotoxins
Polycyclic aromatic hydrocarbons (PAHs) are composed of multiple fused aromatic rings made solely of carbon and hydrogen atoms, exhibiting a planar, non-polar structure that contributes to their high stability and persistence in the environment. Mycotoxins have diverse chemical structures often containing heterocyclic rings and various functional groups such as hydroxyl, carbonyl, or amine groups, imparting different polarities and reactivities depending on the specific toxin. The structural complexity of mycotoxins allows for more biochemical interactions and toxicity mechanisms compared to the simpler, hydrophobic PAHs.
Natural and Anthropogenic Sources
Polycyclic aromatic hydrocarbons (PAHs) primarily originate from incomplete combustion of fossil fuels, biomass burning, and industrial processes, representing significant anthropogenic sources. Conversely, mycotoxins are naturally produced by certain fungi, commonly contaminating crops and stored food under specific environmental conditions. Natural PAH sources include forest fires and volcanic activity, while mycotoxin occurrence is influenced by humidity and temperature in agricultural settings.
Pathways of Human and Environmental Exposure
Polycyclic aromatic hydrocarbons (PAHs) primarily enter the human body through inhalation of polluted air, ingestion of contaminated food, and dermal contact with contaminated soil or water, posing significant environmental health risks due to their persistence and bioaccumulation. Mycotoxins, produced by mold fungi, contaminate food crops such as grains and nuts, leading to human exposure mainly through ingestion, while environmental exposure occurs via contaminated soil and air spores. Both toxicants have distinct pathways yet contribute to notable health hazards through dietary intake and environmental contact, necessitating rigorous monitoring and control measures.
Mechanisms of Toxicity
Polycyclic aromatic hydrocarbons (PAHs) exert toxicity primarily through metabolic activation by cytochrome P450 enzymes, forming reactive intermediates that bind to DNA and induce mutations leading to carcinogenesis. Mycotoxins, such as aflatoxins or ochratoxins, disrupt cellular function by inhibiting protein synthesis, inducing oxidative stress, and triggering immunosuppression or apoptosis. Both toxins initiate cellular damage through distinct biochemical pathways, with PAHs mainly causing genotoxic effects and mycotoxins affecting metabolic and immunological systems.
Occurrence in Food and Environment
Polycyclic aromatic hydrocarbons (PAHs) frequently occur in grilled, smoked, and charred foods due to incomplete combustion of organic matter, and are commonly present in polluted air, soil, and water environments. Mycotoxins, produced by certain fungi like Aspergillus and Fusarium species, contaminate crops such as maize, peanuts, and cereals during pre-harvest, storage, and processing under warm, humid conditions. Both contaminants pose significant risks in food safety, with PAHs linked to environmental pollution and mycotoxins primarily associated with fungal growth in agricultural commodities.
Health Risks Associated with PAHs and Mycotoxins
Polycyclic aromatic hydrocarbons (PAHs) and mycotoxins pose significant health risks primarily through carcinogenic and toxic effects. PAHs, formed by incomplete combustion of organic materials, are linked to lung, skin, and bladder cancers due to their DNA-damaging properties. Mycotoxins, toxic metabolites produced by molds such as Aspergillus and Fusarium species, cause liver damage, immunosuppression, and are associated with acute poisoning and chronic diseases including cancer.
Detection and Analytical Methods
Detection of polycyclic aromatic hydrocarbons (PAHs) primarily involves chromatographic techniques such as gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC) with fluorescence detection, enabling precise quantification of PAH concentrations in environmental and biological samples. Mycotoxin analysis relies heavily on immunoassays like enzyme-linked immunosorbent assay (ELISA) and advanced chromatographic methods including liquid chromatography-tandem mass spectrometry (LC-MS/MS) for accurate detection in food matrices. Both contaminants require meticulous sample preparation and cleanup procedures, with PAHs often necessitating solid-phase extraction (SPE) and mycotoxins demanding methods like immunoaffinity columns to reduce matrix effects and enhance method sensitivity.
Regulatory Standards and Guidelines
Regulatory standards for polycyclic aromatic hydrocarbons (PAHs) focus primarily on limits in food, air, and water, set by agencies such as the US EPA and EU EFSA, with maximum allowable levels often below 1 ug/kg for food products. Mycotoxin regulations, governed by entities like the FDA and Codex Alimentarius, specify maximum permissible levels for compounds like aflatoxins and ochratoxins, typically in the range of 1-20 ug/kg depending on the toxin and commodity. Both PAHs and mycotoxins undergo rigorous risk assessment to establish these guidelines, emphasizing target populations, toxicity profiles, and exposure routes to minimize health hazards.
Strategies for Risk Reduction and Prevention
Strategies for risk reduction and prevention of polycyclic aromatic hydrocarbons (PAHs) focus on minimizing exposure through controlling sources such as industrial emissions, tobacco smoke, and grilled or charred foods, alongside promoting regulations for air and water quality. For mycotoxins, prevention emphasizes proper agricultural practices, moisture control during crop storage, and regular monitoring of food products to inhibit fungal contamination and toxin production. Both hazards benefit from public awareness campaigns and advanced detection technologies to ensure early identification and mitigation of risks.
Polycyclic aromatic hydrocarbon Infographic
 libterm.com
libterm.com