Acidic soil has a pH level below 7, which affects nutrient availability and plant growth by increasing the solubility of harmful metals like aluminum and reducing essential nutrients such as calcium and magnesium. Many plants struggle to thrive in acidic conditions, making soil testing and amendment crucial for healthy gardening or farming. Discover effective strategies to manage acidic soil and improve your garden's productivity in the rest of this article.
Table of Comparison
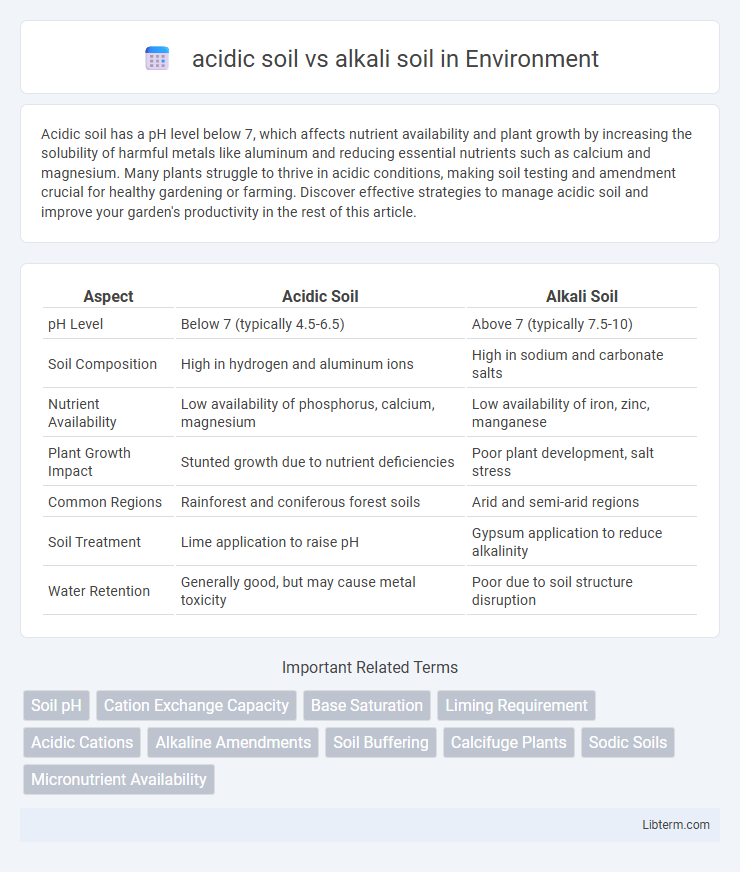
| Aspect | Acidic Soil | Alkali Soil |
|---|---|---|
| pH Level | Below 7 (typically 4.5-6.5) | Above 7 (typically 7.5-10) |
| Soil Composition | High in hydrogen and aluminum ions | High in sodium and carbonate salts |
| Nutrient Availability | Low availability of phosphorus, calcium, magnesium | Low availability of iron, zinc, manganese |
| Plant Growth Impact | Stunted growth due to nutrient deficiencies | Poor plant development, salt stress |
| Common Regions | Rainforest and coniferous forest soils | Arid and semi-arid regions |
| Soil Treatment | Lime application to raise pH | Gypsum application to reduce alkalinity |
| Water Retention | Generally good, but may cause metal toxicity | Poor due to soil structure disruption |
Introduction to Soil pH
Soil pH measures the acidity or alkalinity of soil on a scale from 0 to 14, with 7 being neutral. Acidic soil has a pH below 7, often ranging from 4 to 6, which affects nutrient availability and microbial activity. In contrast, alkaline soil has a pH above 7, typically between 7 and 9, influencing plant growth by altering nutrient solubility and accessibility.
What is Acidic Soil?
Acidic soil is characterized by a pH level below 7, typically ranging from 4 to 6, which affects nutrient availability and microbial activity essential for plant growth. High acidity often results from excessive rainfall, organic matter decomposition, or acid-forming fertilizers, leading to aluminum and manganese toxicity in plants. Managing acidic soil involves liming to raise pH, improving nutrient uptake, and enhancing crop yield and soil health.
What is Alkali Soil?
Alkali soil, also known as sodic soil, contains high levels of sodium ions that cause poor soil structure, leading to reduced aeration and water infiltration. This type of soil typically exhibits a pH greater than 8.5, resulting in nutrient imbalances and toxicity that affect plant growth. Managing alkali soil involves amendments like gypsum and organic matter to displace sodium and improve soil fertility.
Key Differences Between Acidic and Alkali Soils
Acidic soils have a pH below 7, often rich in aluminum and iron, which can limit nutrient availability and affect plant growth, while alkali soils have a pH above 7, characterized by high sodium content that causes poor soil structure and water infiltration. Acidic soils typically occur in regions with high rainfall, leading to leaching of basic ions, whereas alkali soils are common in arid and semi-arid areas where evaporation exceeds precipitation. Managing acidic soils involves liming to increase pH, while alkali soils require gypsum application to displace sodium and improve soil texture.
Causes of Acidic Soil Formation
Acidic soil formation is primarily caused by high rainfall that leaches away basic ions such as calcium, magnesium, and potassium, leaving behind acidic elements like hydrogen and aluminum. The decomposition of organic matter producing organic acids, acid rain resulting from industrial pollution, and excessive use of nitrogenous fertilizers further contribute to soil acidity. These factors collectively reduce soil pH below 7, impacting nutrient availability and microbial activity in the soil.
Causes of Alkali Soil Formation
Alkali soil formation occurs primarily due to the accumulation of sodium carbonate and bicarbonate salts in the soil profile, often resulting from poor drainage and high evaporation rates. Irrigation with sodium-rich water and prolonged weathering of parent materials containing sodium contribute significantly to soil alkalinity. This leads to soil dispersion, poor structure, and reduced fertility, hindering plant growth.
Effects of Soil pH on Plant Growth
Soil pH significantly influences nutrient availability and microbial activity, directly affecting plant growth and health. Acidic soils (pH < 6) often lead to aluminum and manganese toxicity, hindering root development and nutrient uptake, while alkaline soils (pH > 7.5) cause deficiencies in iron, zinc, and phosphorus, resulting in chlorosis and stunted growth. Optimal plant growth generally occurs in neutral to slightly acidic soils (pH 6-7), where essential nutrients are most accessible and microbial processes are balanced.
Indicators and Testing of Soil pH
Soil pH indicators such as litmus paper and pH meters provide precise measurement of hydrogen ion concentration, distinguishing acidic soils (pH less than 7) from alkaline soils (pH greater than 7). Field test kits using chemical reagents change color based on soil pH levels, offering a quick visual assessment of acidity or alkalinity. Laboratory soil testing involves soil sampling and analysis for accurate pH values, essential for effective soil management and amendment strategies.
Management Strategies for Acidic and Alkali Soils
Management strategies for acidic soils include liming with materials such as calcium carbonate or dolomite to raise pH and improve nutrient availability, alongside incorporating organic matter to enhance soil structure and microbial activity. In alkali soils, gypsum application is crucial to replace sodium ions, combined with proper irrigation techniques to leach excess salts and improve soil permeability. Both soil types benefit from selecting tolerant crop varieties and implementing balanced fertilization to optimize nutrient uptake and maintain soil health.
Conclusion: Choosing the Right Soil for Your Crops
Selecting the appropriate soil pH is crucial for maximizing crop yield and health, with acidic soils favoring crops like blueberries and potatoes while alkali soils suit crops such as barley and spinach. Soil amendments, including lime for acidic soils and sulfur for alkaline soils, can effectively adjust pH levels to optimize nutrient availability. Understanding crop-specific pH preferences and managing soil chemistry ensures sustainable agriculture and improved productivity.
acidic soil Infographic
 libterm.com
libterm.com