Chemical oxidation is a process that involves the transfer of electrons, increasing the oxidation state of a substance often through the reaction with oxidizing agents. It plays a crucial role in environmental remediation, organic synthesis, and industrial processes by breaking down contaminants and transforming materials. Explore the rest of the article to understand how chemical oxidation can impact your projects and improve outcomes.
Table of Comparison
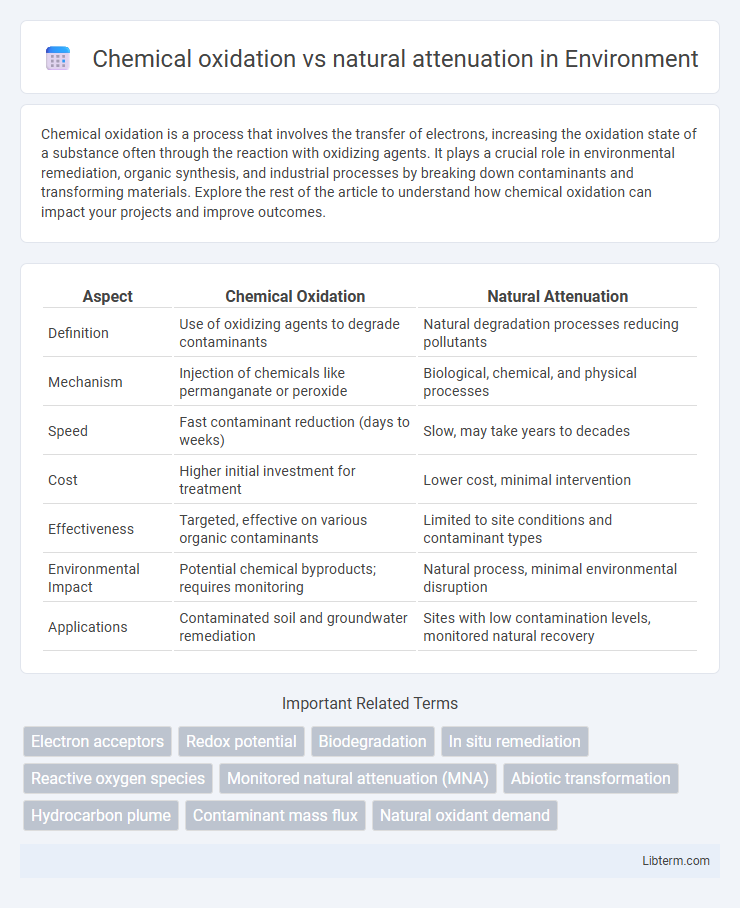
| Aspect | Chemical Oxidation | Natural Attenuation |
|---|---|---|
| Definition | Use of oxidizing agents to degrade contaminants | Natural degradation processes reducing pollutants |
| Mechanism | Injection of chemicals like permanganate or peroxide | Biological, chemical, and physical processes |
| Speed | Fast contaminant reduction (days to weeks) | Slow, may take years to decades |
| Cost | Higher initial investment for treatment | Lower cost, minimal intervention |
| Effectiveness | Targeted, effective on various organic contaminants | Limited to site conditions and contaminant types |
| Environmental Impact | Potential chemical byproducts; requires monitoring | Natural process, minimal environmental disruption |
| Applications | Contaminated soil and groundwater remediation | Sites with low contamination levels, monitored natural recovery |
Introduction to Chemical Oxidation and Natural Attenuation
Chemical oxidation involves the injection or application of oxidizing agents like hydrogen peroxide, potassium permanganate, or ozone to rapidly degrade contaminants in soil or groundwater. Natural attenuation relies on intrinsic environmental processes such as microbial activity, chemical reactions, and physical dispersion to gradually reduce contaminant concentrations without active intervention. Both methods serve as remediation strategies, with chemical oxidation offering faster contaminant destruction while natural attenuation depends on site-specific conditions and longer time frames for effectiveness.
Fundamental Principles of Chemical Oxidation
Chemical oxidation utilizes reactive oxidants such as hydrogen peroxide, potassium permanganate, or ozone to transform contaminants into less harmful compounds by breaking chemical bonds through redox reactions. This remediation method accelerates degradation rates compared to natural attenuation, which relies on intrinsic biogeochemical processes and microbial activity to gradually reduce pollutant concentrations over time. The effectiveness of chemical oxidation depends on oxidant delivery, contaminant chemistry, and site conditions, making it a targeted approach for site-specific contaminant removal.
Core Mechanisms of Natural Attenuation
Natural attenuation relies primarily on biotic and abiotic mechanisms such as biodegradation, adsorption, dilution, volatilization, and chemical stabilization to reduce contaminant concentrations in soil and groundwater. Microbial populations metabolize organic pollutants, transforming them into less harmful substances under aerobic or anaerobic conditions, while physical processes like dispersion and sorption limit contaminant mobility. Chemical oxidation, by contrast, introduces strong oxidants like potassium permanganate or hydrogen peroxide to rapidly degrade contaminants, bypassing the slower, naturally occurring attenuation pathways.
Comparing Effectiveness: Chemical Oxidation vs Natural Attenuation
Chemical oxidation accelerates contaminant breakdown by introducing powerful oxidants, resulting in rapid reduction of pollutants such as chlorinated solvents and petroleum hydrocarbons, often achieving cleanup within months. Natural attenuation relies on inherent biological, chemical, and physical processes, which can be effective for low-concentration contaminants but typically requires years to achieve similar results. Compared to natural attenuation, chemical oxidation provides a more controlled and predictable remediation timeline, crucial for sites with stringent regulatory deadlines or high contamination levels.
Environmental Impact and Sustainability Considerations
Chemical oxidation rapidly degrades contaminants in soil and groundwater but may produce harmful byproducts, impacting local ecosystems and requiring careful management to prevent secondary pollution. Natural attenuation relies on inherent biological and chemical processes to reduce contaminant levels over time, promoting ecosystem recovery with minimal intervention and lower energy consumption. Sustainability considerations favor natural attenuation for long-term environmental health, while chemical oxidation offers accelerated remediation for urgent contamination scenarios.
Site Assessment and Selection Criteria
Chemical oxidation involves the rapid transformation of contaminants through injected oxidants, requiring thorough site assessment including contaminant type, concentration, and subsurface geology to ensure effective delivery and reaction. Natural attenuation relies on inherent biological, chemical, and physical processes to reduce contaminant levels, with site selection criteria emphasizing contaminant biodegradability, presence of native microbial populations, and hydrogeological conditions that support natural degradation pathways. Detailed evaluation of contaminant plume characteristics and site-specific parameters guides the selection of chemical oxidation or natural attenuation as the most feasible remediation strategy.
Implementation and Operational Factors
Chemical oxidation requires careful implementation involving the precise delivery of oxidants such as hydrogen peroxide, potassium permanganate, or ozone directly into the contaminated media, with monitoring to control reaction rates and prevent unwanted side effects like soil heating or mobilization of contaminants. Operational factors include the need for specialized equipment, trained personnel, and ongoing site monitoring to assess oxidant distribution and contaminant breakdown efficiency, often resulting in faster remediation compared to natural attenuation. Natural attenuation relies on inherent microbial, chemical, and physical processes within the environment to degrade contaminants over time, requiring extensive site characterization and long-term monitoring to ensure contaminant levels decrease without active intervention.
Cost Analysis and Economic Implications
Chemical oxidation typically involves higher upfront costs due to the purchase of reagents and specialized equipment, but it can accelerate contaminant degradation, potentially reducing long-term monitoring expenses. Natural attenuation relies on inherent microbial and chemical processes, resulting in lower immediate expenditures but often extending the cleanup timeline, which can increase overall project duration and indirect costs. Cost analyses must weigh short-term investment against prolonged site management, considering factors like contaminant concentration, site conditions, and regulatory requirements to determine economic feasibility.
Regulatory Guidance and Compliance
Regulatory guidance for chemical oxidation mandates thorough site characterization and verification of contaminant reduction to meet Environmental Protection Agency (EPA) standards under the Superfund program. Natural attenuation requires documented evidence of contaminant degradation rates consistent with regulatory benchmarks outlined in the EPA's Monitored Natural Attenuation (MNA) protocols to ensure compliance. Both approaches demand detailed monitoring plans and reporting to state environmental agencies such as the California Regional Water Quality Control Board (RWQCB) for site closure approval.
Future Trends and Innovations in Remediation Technologies
Emerging trends in chemical oxidation emphasize the development of highly reactive, sustainable oxidants such as biochar-activated persulfate and electrochemical oxidation systems that enhance contaminant degradation efficiency while minimizing environmental impact. Natural attenuation increasingly integrates advanced microbial genomics and real-time monitoring technologies to optimize in situ bioremediation, enabling precise prediction and acceleration of contaminant breakdown. Innovations combining chemical oxidation with bioaugmentation and nanomaterials hold significant promise for hybrid remediation approaches, driving future environmental cleanup efforts toward greater effectiveness and cost-efficiency.
Chemical oxidation Infographic
 libterm.com
libterm.com