Absorptivity measures a material's ability to absorb radiation at specific wavelengths, playing a crucial role in fields like material science and environmental engineering. High absorptivity materials are sought after for improving solar energy capture and thermal insulation efficiency. Discover how understanding absorptivity can enhance your design choices by reading the rest of this article.
Table of Comparison
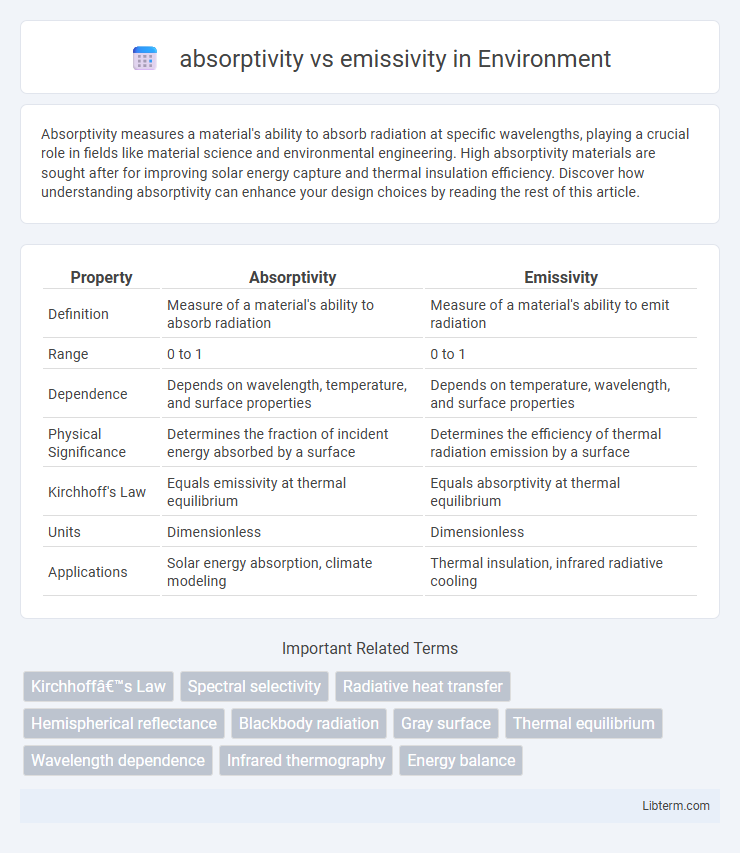
| Property | Absorptivity | Emissivity |
|---|---|---|
| Definition | Measure of a material's ability to absorb radiation | Measure of a material's ability to emit radiation |
| Range | 0 to 1 | 0 to 1 |
| Dependence | Depends on wavelength, temperature, and surface properties | Depends on temperature, wavelength, and surface properties |
| Physical Significance | Determines the fraction of incident energy absorbed by a surface | Determines the efficiency of thermal radiation emission by a surface |
| Kirchhoff's Law | Equals emissivity at thermal equilibrium | Equals absorptivity at thermal equilibrium |
| Units | Dimensionless | Dimensionless |
| Applications | Solar energy absorption, climate modeling | Thermal insulation, infrared radiative cooling |
Understanding Absorptivity: Definition and Significance
Absorptivity refers to the fraction of incident radiation that a material absorbs, directly influencing its thermal performance and energy balance. High absorptivity materials absorb more radiant energy, making them crucial in applications like solar thermal collectors and heat management systems. Understanding absorptivity helps optimize material selection for energy efficiency and thermal regulation by tailoring surface properties to specific radiation wavelengths.
What is Emissivity? Key Concepts Explained
Emissivity is the measure of a material's effectiveness in emitting thermal radiation compared to an ideal black body, with values ranging from 0 to 1. High emissivity surfaces, close to 1, radiate heat efficiently, while low emissivity materials reflect more incident energy and emit less thermal radiation. This property plays a crucial role in heat transfer applications, influencing thermal insulation, radiative cooling, and temperature regulation in engineering and environmental science.
Scientific Basis: How Absorptivity and Emissivity Relate
Absorptivity and emissivity are fundamental radiative properties governed by Kirchhoff's law of thermal radiation, stating that at thermal equilibrium, a body's absorptivity equals its emissivity at each wavelength. These properties quantify how a material absorbs incident radiant energy and emits its own thermal radiation, respectively, influencing heat transfer processes. Measuring absorptivity and emissivity across the electromagnetic spectrum provides critical data for modeling energy exchange in applications such as climate science, thermal insulation, and solar energy systems.
Mathematical Formulations of Absorptivity and Emissivity
Absorptivity (a) and emissivity (e) are dimensionless quantities representing a material's ability to absorb and emit radiant energy, respectively, both ranging from 0 to 1. Mathematically, absorptivity is defined as the ratio of absorbed radiant power (Q_abs) to incident radiant power (Q_inc), expressed as a = Q_abs / Q_inc, while emissivity is defined as the ratio of the radiant energy emitted by the material (E) to that emitted by a blackbody at the same temperature (E_bb), given as e = E / E_bb. Kirchhoff's law of thermal radiation establishes that, under thermal equilibrium, absorptivity equals emissivity (a = e) for a given wavelength and temperature, linking the two properties through their spectral dependence.
Material Properties Influencing Absorptivity and Emissivity
Material properties such as surface roughness, chemical composition, and temperature significantly influence absorptivity and emissivity, determining how materials absorb and emit radiant energy. Metals typically exhibit low absorptivity and high reflectivity, resulting in lower emissivity, while non-metals and oxidized surfaces show higher emissivity due to increased absorption and radiation capabilities. Temperature changes alter vibrational states and electronic structures, thereby affecting a material's ability to absorb and emit thermal radiation efficiently.
Measurement Techniques for Absorptivity and Emissivity
Measurement techniques for absorptivity and emissivity involve using spectrophotometers and radiometers to capture wavelength-specific data, enabling precise characterization of material properties. Integrating spheres and Fourier-transform infrared (FTIR) spectroscopy are commonly employed to measure hemispherical reflectance and transmittance, which are essential for calculating absorptivity and emissivity values accurately. Calibrated blackbody sources serve as reference standards in emissivity assessments, ensuring reliable thermal emission measurements under controlled environmental conditions.
Practical Applications: Engineering and Industry Examples
In engineering and industrial applications, absorptivity and emissivity significantly impact thermal management and energy efficiency. Solar panels utilize materials with high absorptivity to maximize solar energy capture, while radiative cooling surfaces are designed with high emissivity to efficiently dissipate heat. Thermal insulation systems balance low absorptivity and low emissivity to minimize heat transfer, enhancing temperature control in buildings and manufacturing processes.
Absorptivity vs Emissivity in Thermal Radiation
Absorptivity quantifies a material's ability to absorb incident thermal radiation, while emissivity measures its efficiency in emitting thermal radiation relative to a perfect blackbody. Kirchhoff's law of thermal radiation establishes that, at thermal equilibrium, the absorptivity and emissivity of a material are equal at each wavelength and temperature. High absorptivity materials typically exhibit high emissivity, influencing heat transfer processes in applications such as solar energy harvesting and thermal insulation.
Common Misconceptions and Challenges
Absorptivity and emissivity are often mistakenly assumed to be identical; however, absorptivity measures a material's ability to absorb incident radiation, while emissivity quantifies how efficiently it emits thermal radiation at a given temperature. A common challenge in thermal analysis is that materials with high absorptivity do not always possess high emissivity, complicating accurate heat transfer modeling in applications like solar energy systems and thermal insulation. Misunderstandings in these properties can lead to design inefficiencies, especially when selecting coatings or materials for radiative heat exchange.
Summary Table: Comparing Absorptivity and Emissivity
Absorptivity measures a material's ability to absorb incident radiation, while emissivity quantifies its efficiency in emitting thermal radiation. Both properties are dimensionless, ranging from 0 to 1, with values closer to 1 indicating higher absorption or emission capacity. The summary table compares key materials: black surfaces exhibit high absorptivity (~0.95) and emissivity (~0.95), metals show low absorptivity (~0.1) and emissivity (~0.1), and polished surfaces have significantly lower values for both properties.
absorptivity Infographic
 libterm.com
libterm.com