Acid mine drainage occurs when sulfide minerals in exposed rock surfaces react with air and water, producing sulfuric acid that contaminates nearby streams and groundwater. This acidic runoff mobilizes heavy metals, posing serious environmental and health risks. Discover how acid mine drainage forms, its impacts, and effective remediation strategies in the rest of the article.
Table of Comparison
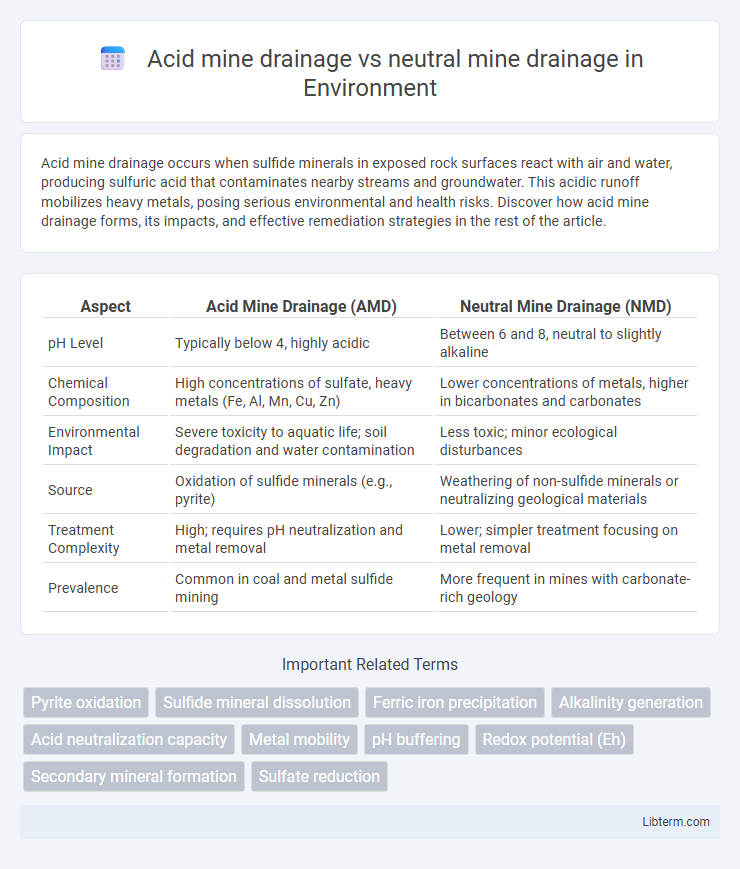
| Aspect | Acid Mine Drainage (AMD) | Neutral Mine Drainage (NMD) |
|---|---|---|
| pH Level | Typically below 4, highly acidic | Between 6 and 8, neutral to slightly alkaline |
| Chemical Composition | High concentrations of sulfate, heavy metals (Fe, Al, Mn, Cu, Zn) | Lower concentrations of metals, higher in bicarbonates and carbonates |
| Environmental Impact | Severe toxicity to aquatic life; soil degradation and water contamination | Less toxic; minor ecological disturbances |
| Source | Oxidation of sulfide minerals (e.g., pyrite) | Weathering of non-sulfide minerals or neutralizing geological materials |
| Treatment Complexity | High; requires pH neutralization and metal removal | Lower; simpler treatment focusing on metal removal |
| Prevalence | Common in coal and metal sulfide mining | More frequent in mines with carbonate-rich geology |
Introduction to Mine Drainage
Mine drainage refers to the outflow of water from mining sites, which can be classified primarily as Acid Mine Drainage (AMD) or Neutral Mine Drainage (NMD). Acid Mine Drainage occurs when sulfide minerals exposed during mining react with oxygen and water, producing sulfuric acid that lowers pH and mobilizes heavy metals. Neutral Mine Drainage, in contrast, maintains near-neutral pH levels due to the presence of carbonate minerals that neutralize acidity, resulting in less environmental impact compared to AMD.
Defining Acid Mine Drainage (AMD)
Acid Mine Drainage (AMD) occurs when sulfide minerals, primarily pyrite (FeS2), oxidize and produce sulfuric acid, leading to highly acidic water with pH values often below 4. This acidic water leaches heavy metals such as iron, copper, and arsenic, severely impacting aquatic ecosystems. In contrast, neutral mine drainage typically retains near-neutral pH levels and contains lower concentrations of dissolved metals, posing less environmental risk.
Understanding Neutral Mine Drainage (NMD)
Neutral Mine Drainage (NMD) occurs when mining activities generate water with near-neutral pH, typically between 6 and 8, minimizing the release of heavy metals compared to Acid Mine Drainage (AMD), which is highly acidic. NMD often forms in environments with abundant carbonate minerals, which neutralize acidity through chemical buffering, reducing environmental toxicity and metal solubility. Understanding NMD's chemical characteristics helps optimize mine water management and remediation strategies that prevent harmful metal mobilization in mining-impacted ecosystems.
Causes and Formation Processes
Acid mine drainage (AMD) forms when sulfide minerals, especially pyrite (FeS2), are exposed to air and water, triggering oxidation and producing sulfuric acid that lowers pH levels and mobilizes heavy metals. Neutral mine drainage (NMD) occurs when carbonate minerals like calcite neutralize acidity during mineral oxidation, leading to near-neutral pH waters with fewer dissolved metals. The presence and balance of sulfide and carbonate minerals primarily dictate whether mine drainage becomes acidic or remains neutral.
Chemical Composition Differences
Acid mine drainage (AMD) typically contains high concentrations of sulfuric acid and dissolved metals such as iron, aluminum, and heavy metals like arsenic and lead, resulting from the oxidation of sulfide minerals. Neutral mine drainage (NMD) generally has near-neutral pH levels and lower concentrations of dissolved metals due to the presence of carbonate minerals that neutralize acidity. The chemical composition differences directly influence the toxicity and environmental impact of each drainage type, with AMD posing greater risks due to its acidity and metal solubility.
Environmental Impacts: AMD vs NMD
Acid mine drainage (AMD) results in highly acidic water with elevated concentrations of heavy metals like iron, copper, and lead, severely harming aquatic life and contaminating drinking water sources. Neutral mine drainage (NMD) typically exhibits near-neutral pH, reducing metal solubility and posing less immediate toxicity, yet it can still transport metals and sulfates that impact ecosystems. The environmental impact of AMD is generally more severe due to its acidity and metal bioavailability, while NMD requires monitoring to mitigate long-term contamination risks.
Effects on Aquatic and Terrestrial Ecosystems
Acid mine drainage (AMD) severely lowers pH levels, releasing toxic metals such as iron, aluminum, and heavy metals that devastate aquatic life by causing fish kills and disrupting reproductive cycles. Neutral mine drainage (NMD) has a higher pH, often near neutral, and while it contains fewer acids, it can still introduce elevated metal concentrations that accumulate in sediments, impacting benthic organisms and terrestrial food webs. Both AMD and NMD alter nutrient dynamics and habitat quality, leading to reduced biodiversity and long-term ecosystem degradation in affected aquatic and terrestrial environments.
Detection and Monitoring Techniques
Acid mine drainage (AMD) and neutral mine drainage (NMD) require distinct detection and monitoring techniques due to differences in pH and contaminant profiles. AMD is commonly detected using pH sensors, metal ion analyzers, and sulfate concentration measurements to monitor its high acidity and heavy metal levels, while NMD monitoring emphasizes metals and neutral pH stability using spectroscopic methods and ion chromatography. Real-time remote sensing combined with automated sampling systems enhances the continuous assessment of both AMD and NMD, optimizing environmental impact evaluations.
Treatment and Remediation Strategies
Acid mine drainage (AMD) treatment focuses on neutralizing highly acidic waters using alkaline materials such as lime or limestone to raise pH and precipitate heavy metals, often requiring active treatment systems like chemical dosing and aeration. Neutral mine drainage, characterized by near-neutral pH, typically necessitates passive treatment methods including constructed wetlands, bioreactors, and permeable reactive barriers that harness natural attenuation processes to remove contaminants. Both AMD and neutral drainage remediation strategies prioritize metal removal and water quality improvement, but differ in complexity and operational cost due to the acidity level and chemical composition of the mine drainage.
Prevention and Mitigation Best Practices
Effective prevention of acid mine drainage (AMD) centers on controlling sulfide mineral oxidation through measures like water diversion, alkaline material addition, and proper waste rock management to inhibit acid generation. Neutral mine drainage (NMD) management focuses on preventing metal mobilization and promoting natural attenuation by maintaining neutral pH conditions using constructed wetlands, controlled water flow, and vegetative covers. Both drainage types benefit from integrated monitoring systems and adaptive remediation techniques to minimize environmental impacts and ensure long-term containment.
Acid mine drainage Infographic
 libterm.com
libterm.com