Ammonification is the process by which organic nitrogen compounds are decomposed by microorganisms into ammonia or ammonium ions, playing a vital role in the nitrogen cycle. This transformation enriches soil fertility by converting nitrogen from dead plants and animal matter into forms accessible to plants. Discover more about how ammonification impacts your ecosystem and agriculture in the following article.
Table of Comparison
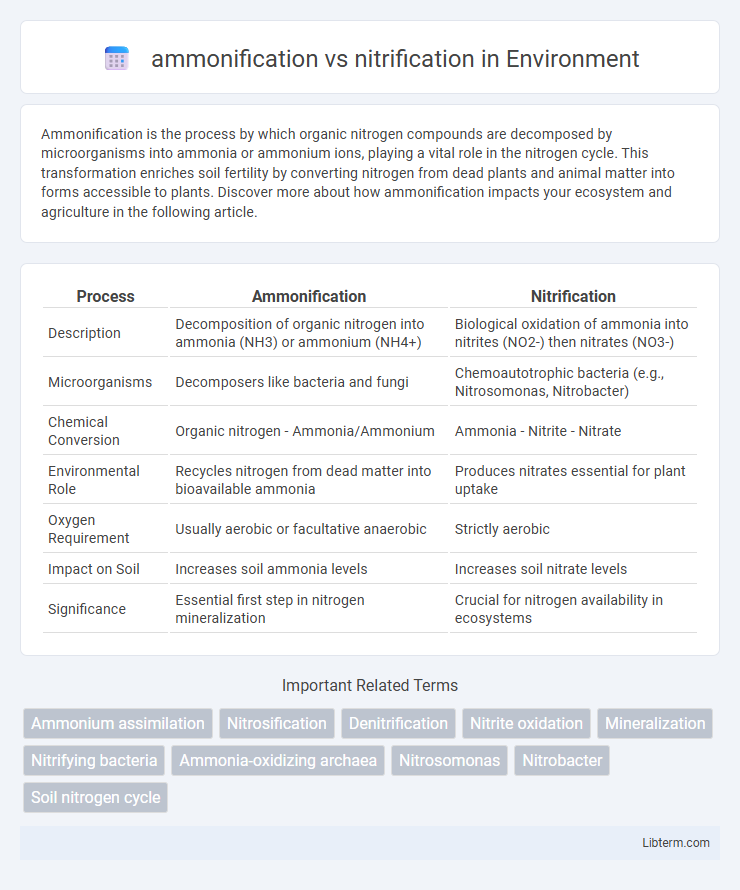
| Process | Ammonification | Nitrification |
|---|---|---|
| Description | Decomposition of organic nitrogen into ammonia (NH3) or ammonium (NH4+) | Biological oxidation of ammonia into nitrites (NO2-) then nitrates (NO3-) |
| Microorganisms | Decomposers like bacteria and fungi | Chemoautotrophic bacteria (e.g., Nitrosomonas, Nitrobacter) |
| Chemical Conversion | Organic nitrogen - Ammonia/Ammonium | Ammonia - Nitrite - Nitrate |
| Environmental Role | Recycles nitrogen from dead matter into bioavailable ammonia | Produces nitrates essential for plant uptake |
| Oxygen Requirement | Usually aerobic or facultative anaerobic | Strictly aerobic |
| Impact on Soil | Increases soil ammonia levels | Increases soil nitrate levels |
| Significance | Essential first step in nitrogen mineralization | Crucial for nitrogen availability in ecosystems |
Introduction to Nitrogen Cycle Processes
Ammonification converts organic nitrogen from dead organisms and waste into ammonia, releasing it into the soil. Nitrification is a two-step microbial process where ammonia is oxidized first to nitrite by Nitrosomonas bacteria and then to nitrate by Nitrobacter bacteria, making nitrogen available for plant uptake. Both processes are essential components of the nitrogen cycle, facilitating nitrogen transformation and recycling in ecosystems.
Definition of Ammonification
Ammonification is the microbial process of decomposing organic nitrogen from dead organisms and waste products into ammonia (NH3) or ammonium ions (NH4+). This stage is essential in the nitrogen cycle, converting complex nitrogenous compounds into simpler forms available for further transformation. Nitrification subsequently oxidizes these ammonium ions into nitrites (NO2-) and nitrates (NO3-) through specialized bacteria, facilitating nutrient uptake by plants.
Definition of Nitrification
Nitrification is a critical biochemical process in the nitrogen cycle where ammonia is oxidized into nitrites and then nitrates by specialized bacteria such as Nitrosomonas and Nitrobacter. This aerobic process converts toxic ammonia into nitrate, a form readily absorbed by plants, enhancing soil fertility and ecosystem productivity. Unlike ammonification, which involves the decomposition of organic nitrogen into ammonia, nitrification plays a pivotal role in nitrogen transformation and availability in terrestrial and aquatic systems.
Key Differences Between Ammonification and Nitrification
Ammonification is the microbial process that converts organic nitrogen from dead organisms and waste into ammonia or ammonium ions, playing a crucial role in the nitrogen cycle by recycling nitrogen in soil. Nitrification is a two-step aerobic process in which specialized bacteria like Nitrosomonas oxidize ammonia into nitrites, followed by Nitrobacter converting nitrites into nitrates, making nitrogen available for plant uptake. The key difference lies in ammonification producing ammonia, while nitrification transforms ammonia into nitrites and nitrates, facilitating nitrogen assimilation in ecosystems.
Microorganisms Involved in Ammonification
Ammonification is primarily driven by decomposer microorganisms such as saprophytic bacteria and fungi, which break down organic nitrogen from dead organisms and waste products into ammonia or ammonium ions. Key bacterial genera involved include Bacillus, Clostridium, and Proteus species, playing a critical role in nitrogen recycling within soil ecosystems. These ammonifying microbes create essential substrates for nitrification, where distinct nitrifying bacteria like Nitrosomonas and Nitrobacter subsequently convert ammonia into nitrites and nitrates.
Microorganisms Involved in Nitrification
Nitrification primarily involves two groups of microorganisms: ammonia-oxidizing bacteria (AOB) such as Nitrosomonas, which convert ammonia (NH3) into nitrite (NO2-), and nitrite-oxidizing bacteria (NOB) like Nitrobacter, which oxidize nitrite into nitrate (NO3-). These chemolithoautotrophic bacteria play a crucial role in the nitrogen cycle by transforming ammonia released during ammonification into nitrate, which plants can readily assimilate. The efficiency of nitrification depends on environmental factors affecting the activity and population dynamics of these microbes.
Chemical Reactions of Ammonification
Ammonification is the microbial process that converts organic nitrogen from dead organisms and waste products into ammonia (NH3) or ammonium ions (NH4+), primarily through enzymatic decomposition. This chemical reaction involves the breakdown of proteins and nucleic acids by decomposer bacteria and fungi, releasing ammonia as an intermediate nitrogen form. In contrast, nitrification is a subsequent aerobic process where ammonia is oxidized first to nitrite (NO2-) and then to nitrate (NO3-) by specialized bacteria such as Nitrosomonas and Nitrobacter.
Chemical Reactions of Nitrification
Nitrification involves a two-step chemical process where ammonia (NH3) is first oxidized to nitrite (NO2-) by ammonia-oxidizing bacteria such as Nitrosomonas, followed by the oxidation of nitrite to nitrate (NO3-) by nitrite-oxidizing bacteria like Nitrobacter. The overall chemical reactions can be summarized as NH3 + 1.5 O2 - NO2- + H+ + H2O and NO2- + 0.5 O2 - NO3-. These aerobic reactions are crucial for converting toxic ammonia into nitrate, a more accessible nitrogen form for plant uptake in the nitrogen cycle.
Ecological Importance of Ammonification and Nitrification
Ammonification converts organic nitrogen from dead organisms and waste into ammonia, replenishing soil nitrogen essential for plant growth, while nitrification transforms this ammonia into nitrates, which are more accessible forms of nitrogen for plants. These processes maintain the nitrogen cycle's balance, supporting ecosystem productivity and soil fertility. Efficient ammonification and nitrification reduce nitrogen pollution and enhance nutrient availability, crucial for sustaining biodiversity and agricultural systems.
Ammonification vs Nitrification: Comparative Table
Ammonification converts organic nitrogen from dead organisms and waste into ammonium (NH4+), primarily facilitated by decomposer bacteria and fungi in soil environments. Nitrification involves a two-step aerobic process where ammonium is oxidized to nitrite (NO2-) by Nitrosomonas bacteria and then to nitrate (NO3-) by Nitrobacter species, crucial for plant-available nitrogen. The comparative table highlights key differences: ammonification releases ammonium directly increasing soil nitrogen, whereas nitrification transforms ammonium into nitrate, affecting soil pH and nitrogen mobility.
ammonification Infographic
 libterm.com
libterm.com