Acid rain results from the emission of sulfur dioxide and nitrogen oxides into the atmosphere, which mix with water vapor to form acidic precipitation. This phenomenon causes significant environmental damage, including the destruction of forests, harm to aquatic life, and corrosion of buildings. Discover how acid rain impacts your environment and what measures can help reduce its harmful effects in the rest of this article.
Table of Comparison
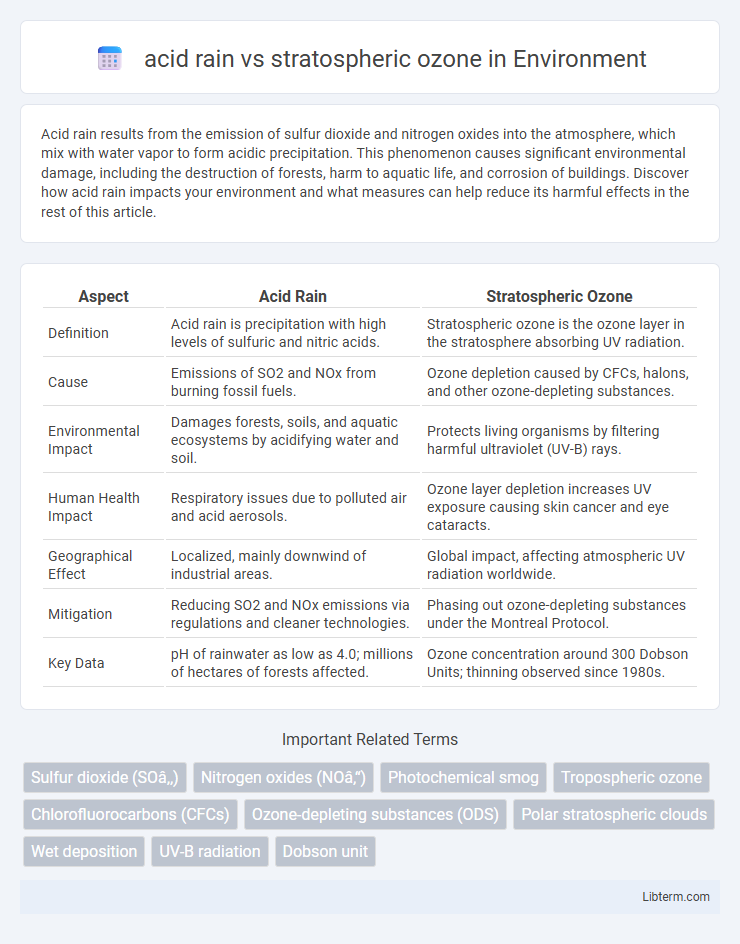
| Aspect | Acid Rain | Stratospheric Ozone |
|---|---|---|
| Definition | Acid rain is precipitation with high levels of sulfuric and nitric acids. | Stratospheric ozone is the ozone layer in the stratosphere absorbing UV radiation. |
| Cause | Emissions of SO2 and NOx from burning fossil fuels. | Ozone depletion caused by CFCs, halons, and other ozone-depleting substances. |
| Environmental Impact | Damages forests, soils, and aquatic ecosystems by acidifying water and soil. | Protects living organisms by filtering harmful ultraviolet (UV-B) rays. |
| Human Health Impact | Respiratory issues due to polluted air and acid aerosols. | Ozone layer depletion increases UV exposure causing skin cancer and eye cataracts. |
| Geographical Effect | Localized, mainly downwind of industrial areas. | Global impact, affecting atmospheric UV radiation worldwide. |
| Mitigation | Reducing SO2 and NOx emissions via regulations and cleaner technologies. | Phasing out ozone-depleting substances under the Montreal Protocol. |
| Key Data | pH of rainwater as low as 4.0; millions of hectares of forests affected. | Ozone concentration around 300 Dobson Units; thinning observed since 1980s. |
Introduction to Acid Rain and Stratospheric Ozone
Acid rain forms when sulfur dioxide (SO2) and nitrogen oxides (NOx) emitted by burning fossil fuels mix with atmospheric moisture, leading to harmful precipitation that damages ecosystems and structures. Stratospheric ozone, concentrated in the ozone layer between 15 and 35 kilometers above Earth's surface, plays a crucial role by absorbing the majority of the sun's harmful ultraviolet (UV) radiation. Understanding the chemical processes behind acid rain and the protective function of stratospheric ozone is essential for environmental management and atmospheric science.
Formation Processes: Acid Rain vs Stratospheric Ozone Depletion
Acid rain forms from sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions that react with water vapor in the lower atmosphere, producing sulfuric and nitric acids. Stratospheric ozone depletion occurs when chlorofluorocarbons (CFCs) release chlorine atoms under UV radiation, which catalytically break down ozone (O3) molecules in the stratosphere. The contrasting chemical processes highlight that acid rain is mainly driven by pollutant interactions in the troposphere, while ozone depletion involves halogen-induced catalytic reactions in the stratosphere.
Major Pollutants Involved
Major pollutants involved in acid rain primarily include sulfur dioxide (SO2) and nitrogen oxides (NOx), which react with water vapor and oxygen in the atmosphere to form sulfuric and nitric acids. In contrast, stratospheric ozone depletion is mainly caused by chlorofluorocarbons (CFCs), halons, and other ozone-depleting substances (ODS) that release chlorine and bromine atoms, catalyzing the breakdown of ozone molecules. While acid rain results from secondary pollutants formed through reactions in the lower atmosphere, stratospheric ozone depletion involves long-lived halogenated compounds affecting the upper atmosphere.
Key Chemical Reactions
Acid rain primarily forms through the oxidation of sulfur dioxide (SO2) and nitrogen oxides (NOx) into sulfuric acid (H2SO4) and nitric acid (HNO3) in the atmosphere, involving reactions such as SO2 + OH - HOSO2 and NO2 + OH - HNO3. Stratospheric ozone depletion centers on catalytic cycles driven by chlorine and bromine radicals, with key reactions including Cl + O3 - ClO + O2 and ClO + O - Cl + O2, which destroy ozone molecules. Both processes illustrate how atmospheric chemistry governs environmental impacts, with acid rain altering terrestrial and aquatic systems, while stratospheric reactions affect UV radiation reaching Earth's surface.
Environmental Impacts of Acid Rain
Acid rain, primarily caused by sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions from fossil fuel combustion, severely damages aquatic ecosystems by lowering water pH and harming fish populations. It accelerates soil acidification, leading to nutrient depletion and forest decline, impacting biodiversity and agricultural productivity. Unlike stratospheric ozone depletion, which mainly increases ultraviolet radiation exposure, acid rain directly alters terrestrial and aquatic habitats, disrupting ecosystem balance and reducing overall environmental health.
Effects of Stratospheric Ozone Depletion
Stratospheric ozone depletion increases the penetration of harmful ultraviolet-B (UV-B) radiation to Earth's surface, leading to higher risks of skin cancer, cataracts, and immune system suppression in humans. It also disrupts marine ecosystems by damaging phytoplankton, the base of aquatic food webs, and impairs terrestrial plant growth, affecting agricultural productivity. Unlike acid rain, which primarily damages forests and aquatic environments through acidification, ozone depletion directly affects UV exposure and global health.
Human Health Consequences
Acid rain primarily causes respiratory problems such as asthma and bronchitis by increasing airborne particulate matter and irritating the mucous membranes. Stratospheric ozone depletion elevates ultraviolet (UV) radiation exposure, significantly raising the risk of skin cancer, cataracts, and immune system suppression. Both environmental issues pose serious threats to human health, but their mechanisms and impact areas differ significantly.
Global and Regional Differences
Acid rain primarily impacts regions with high industrial emissions such as Eastern North America, Europe, and parts of Asia, where sulfur dioxide (SO2) and nitrogen oxides (NOx) from fossil fuel combustion lead to localized acidification of lakes and soils. Stratospheric ozone depletion exhibits a global pattern with pronounced seasonal ozone holes over Antarctica and Arctic regions caused by chlorofluorocarbons (CFCs) and halons, affecting ultraviolet radiation levels worldwide. Regional differences arise because acid rain's effects are concentrated near pollution sources, while stratospheric ozone depletion affects broader atmospheric layers with variations in UV exposure across latitudes.
Prevention and Mitigation Strategies
Reducing sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions from industrial sources and vehicles is essential for preventing acid rain formation by limiting acid precursors in the atmosphere. Stratospheric ozone depletion can be mitigated by phasing out chlorofluorocarbons (CFCs) and other ozone-depleting substances (ODS) through international agreements like the Montreal Protocol. Implementing clean energy technologies and enhancing atmospheric monitoring systems supports both acid rain reduction and ozone layer protection efforts.
Future Challenges and Research Directions
Future challenges in acid rain research involve understanding the interplay between changing industrial emissions and climate-driven alterations in atmospheric chemistry, which affect acidic deposition patterns globally. Stratospheric ozone studies focus on monitoring recovery trends post-Montreal Protocol and investigating the impact of emerging pollutants like short-lived climate forcers on ozone layer stability. Advancing satellite remote sensing, coupled with atmospheric modeling, remains critical for predicting regional acid rain fluctuations and the resilience of the stratospheric ozone layer under evolving environmental policies.
acid rain Infographic
 libterm.com
libterm.com