Acidification refers to the increase in acidity of natural environments, particularly in oceans and soils, due to the absorption of excess carbon dioxide from the atmosphere. This process disrupts marine ecosystems, harms aquatic life, and can lead to soil degradation affecting agriculture and biodiversity. Explore the full article to understand how acidification impacts your environment and what steps can be taken to mitigate its effects.
Table of Comparison
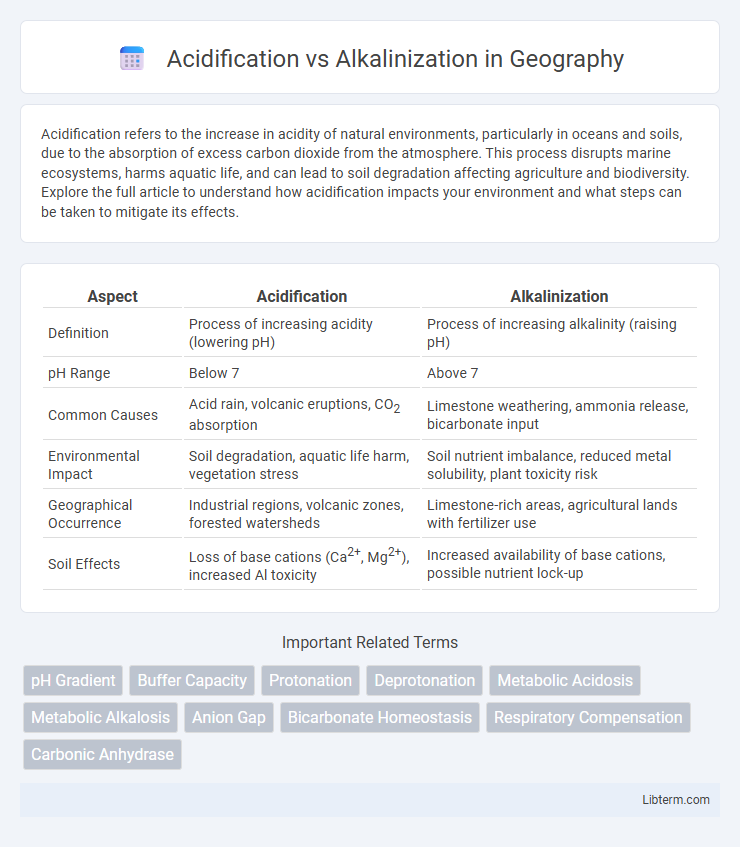
| Aspect | Acidification | Alkalinization |
|---|---|---|
| Definition | Process of increasing acidity (lowering pH) | Process of increasing alkalinity (raising pH) |
| pH Range | Below 7 | Above 7 |
| Common Causes | Acid rain, volcanic eruptions, CO2 absorption | Limestone weathering, ammonia release, bicarbonate input |
| Environmental Impact | Soil degradation, aquatic life harm, vegetation stress | Soil nutrient imbalance, reduced metal solubility, plant toxicity risk |
| Geographical Occurrence | Industrial regions, volcanic zones, forested watersheds | Limestone-rich areas, agricultural lands with fertilizer use |
| Soil Effects | Loss of base cations (Ca2+, Mg2+), increased Al toxicity | Increased availability of base cations, possible nutrient lock-up |
Introduction to Acidification and Alkalinization
Acidification refers to the process by which a substance becomes more acidic, characterized by an increase in hydrogen ion concentration (H+), resulting in a lower pH value. Alkalinization, on the other hand, is the process that increases the substance's alkalinity, marked by a decrease in hydrogen ion concentration and a higher pH. These processes are critical in environmental sciences, physiology, and chemistry, influencing soil health, water quality, and cellular function.
Understanding pH Balance
pH balance is a critical measure of acidity or alkalinity in a solution, ranging from 0 (highly acidic) to 14 (highly alkaline) with 7 being neutral. Understanding the effects of acidification, which lowers pH causing increased hydrogen ion concentration, and alkalinization, which raises pH by reducing hydrogen ions, is essential for maintaining optimal biological and environmental conditions. Proper pH balance supports enzyme function, nutrient availability, and overall system stability in both living organisms and ecosystems.
Causes of Acidification
Acidification primarily results from the excessive release of carbon dioxide (CO2) into the atmosphere, which dissolves in water bodies forming carbonic acid, lowering pH levels. Industrial emissions, deforestation, and the burning of fossil fuels significantly contribute to increased atmospheric CO2, intensifying ocean and soil acidification. Other causes include acid rain caused by sulfur dioxide (SO2) and nitrogen oxides (NOx) from vehicle exhausts and power plants, leading to the acidification of terrestrial and aquatic ecosystems.
Causes of Alkalinization
Alkalinization occurs primarily due to excessive loss of hydrogen ions or an increased intake of alkaline substances such as bicarbonates and antacids. Common causes include vomiting, which leads to loss of gastric acid, diuretic use causing increased renal excretion of hydrogen ions, and excessive consumption of alkaline medications or mineral-rich water. Renal dysfunction can also contribute by impairing acid excretion, further elevating blood pH levels.
Impacts on Human Health
Acidification in the human body, often caused by excess consumption of acidic foods or metabolic imbalances, can lead to symptoms such as fatigue, headaches, and digestive issues, while chronic acidosis may contribute to osteoporosis and kidney stones. Alkalinization, resulting from excessive intake of alkaline substances or disorders like metabolic alkalosis, can cause muscle twitching, irritability, and nausea, with severe cases leading to convulsions or arrhythmias. Maintaining balanced blood pH within the narrow range of 7.35 to 7.45 is critical for enzyme function, oxygen transport, and overall metabolic stability, highlighting the importance of dietary and medical interventions in preventing acid-base disorders.
Effects on Environmental Systems
Acidification causes a decline in pH levels, leading to harmful effects such as coral reef degradation and soil nutrient imbalances, which disrupt aquatic and terrestrial ecosystems. Alkalinization increases pH, often resulting in reduced biodiversity and altered microbial activity, impairing nutrient cycling and plant growth. Both processes impact freshwater quality and soil health, influencing the survival of sensitive species and overall ecosystem stability.
Comparing Chemical and Biological Mechanisms
Acidification involves increasing hydrogen ion concentration, lowering pH through processes like carbon dioxide dissolution or organic acid production in soils and aquatic systems. Alkalinization raises pH by consuming hydrogen ions or releasing hydroxide ions, often driven by mechanisms such as bicarbonate accumulation or ammonia oxidation in biological environments. Chemical acidification typically results from acid rain or industrial emissions, whereas biological acidification and alkalinization stem from microbial metabolism, respiration, and nutrient cycling influencing ecosystem pH dynamics.
Testing and Measuring pH Levels
Testing and measuring pH levels involve using pH meters or indicator strips to determine the acidity or alkalinity of a solution, with values below 7 indicating acidification and above 7 indicating alkalinization. Accurate pH measurement is essential in environmental monitoring, agriculture, and medical diagnostics to assess soil health, water quality, and bodily fluid balance. Calibration of pH meters with standard buffer solutions ensures precision in detecting subtle changes in hydrogen ion concentration during acidification or alkalinization processes.
Prevention and Mitigation Strategies
Effective prevention and mitigation strategies for acidification involve reducing emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) through cleaner energy sources and implementing strict air quality regulations. Alkalinization efforts often focus on soil amendment techniques, such as lime application, to neutralize acidic conditions and restore pH balance in agricultural and aquatic ecosystems. Monitoring pH levels regularly and adopting sustainable land management practices help maintain ecosystem health and prevent long-term environmental damage.
Future Trends and Research Directions
Future trends in acidification and alkalinization research emphasize the development of advanced materials and technologies for precise pH modulation in agriculture, water treatment, and medical applications. Researchers are exploring bioengineered enzymes and nanomaterials to enhance the efficiency and sustainability of pH regulation processes. Cutting-edge studies focus on integrating real-time monitoring systems with AI-driven models to predict and control environmental and biological pH changes more effectively.
Acidification Infographic
 libterm.com
libterm.com