Eutrophication occurs when excessive nutrients, such as nitrogen and phosphorus, accumulate in water bodies, leading to overgrowth of algae and depletion of oxygen. This process disrupts aquatic ecosystems, causing fish kills and loss of biodiversity. Discover how eutrophication impacts your environment and what measures can prevent it by reading the full article.
Table of Comparison
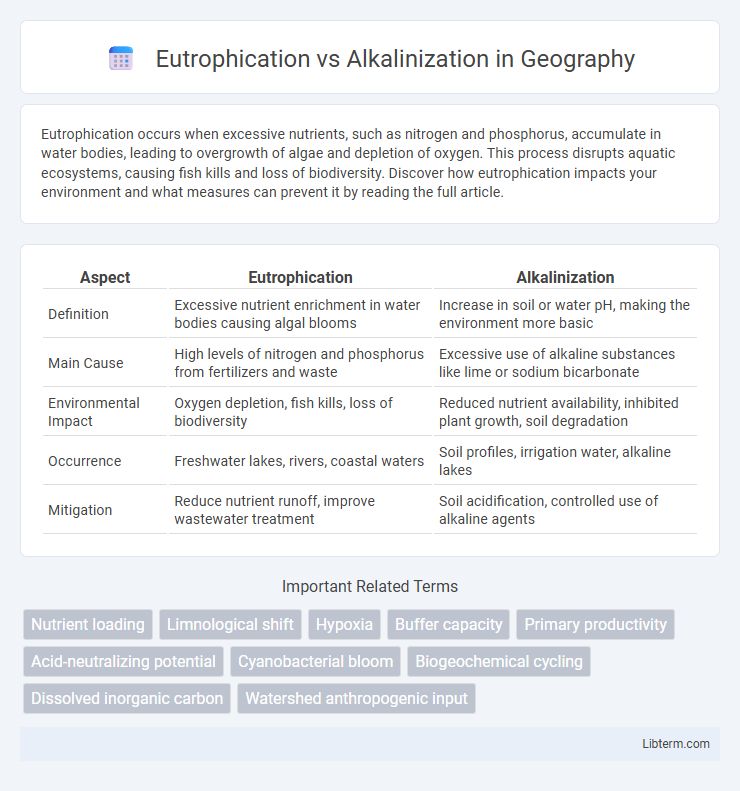
| Aspect | Eutrophication | Alkalinization |
|---|---|---|
| Definition | Excessive nutrient enrichment in water bodies causing algal blooms | Increase in soil or water pH, making the environment more basic |
| Main Cause | High levels of nitrogen and phosphorus from fertilizers and waste | Excessive use of alkaline substances like lime or sodium bicarbonate |
| Environmental Impact | Oxygen depletion, fish kills, loss of biodiversity | Reduced nutrient availability, inhibited plant growth, soil degradation |
| Occurrence | Freshwater lakes, rivers, coastal waters | Soil profiles, irrigation water, alkaline lakes |
| Mitigation | Reduce nutrient runoff, improve wastewater treatment | Soil acidification, controlled use of alkaline agents |
Introduction to Eutrophication and Alkalinization
Eutrophication is the process where water bodies become enriched with excess nutrients, primarily nitrogen and phosphorus, leading to excessive algal growth and oxygen depletion. Alkalinization refers to the increase in pH levels of soil or water, often caused by natural processes or human activities such as liming or industrial pollution. Both phenomena significantly impact aquatic ecosystems, altering chemical balances and affecting biodiversity.
Defining Eutrophication: Causes and Consequences
Eutrophication is the process by which excessive nutrients, primarily nitrogen and phosphorus from agricultural runoff, sewage, and industrial discharges, accumulate in water bodies, leading to dense algal blooms and oxygen depletion. This nutrient overload disrupts aquatic ecosystems, causing hypoxia, fish kills, and loss of biodiversity. Unlike alkalinization, which involves increasing pH levels and can alter water chemistry, eutrophication primarily drives ecosystem imbalances through nutrient enrichment and can result in harmful biological and chemical shifts in freshwater and marine environments.
Understanding Alkalinization: Key Factors and Effects
Alkalinization refers to the increase in pH levels in water bodies, often caused by the influx of alkaline substances such as bicarbonates, carbonates, and hydroxides from natural processes or industrial discharges. Key factors influencing alkalinization include geological formations rich in alkaline minerals, agricultural runoff containing lime, and wastewater effluents with high pH. Elevated alkalinity impacts aquatic ecosystems by altering nutrient availability, disrupting species composition, and potentially reducing the solubility of toxic metals, thereby affecting overall water quality and biodiversity.
Sources of Nutrient Pollution in Aquatic Systems
Eutrophication primarily results from excessive inputs of nutrients such as nitrogen and phosphorus from agricultural runoff, wastewater discharge, and industrial effluents, which stimulate algal blooms and deplete oxygen in aquatic ecosystems. Alkalinization arises from increased concentrations of alkaline substances, including bicarbonates and carbonates, often due to industrial pollution, excessive use of lime in soil, and altered carbon dioxide absorption. Both processes disrupt aquatic system balance, with nutrient pollution from fertilizers and sewage being the dominant source of eutrophication, while alkalinization is linked to chemical imbalances influenced by anthropogenic activities.
Chemical Processes Behind Eutrophication
Eutrophication involves the excessive enrichment of water bodies with nutrients, primarily nitrogen and phosphorus, which accelerates algal blooms through enhanced photosynthesis and biomass accumulation. This process leads to oxygen depletion as microbial decomposition of organic matter consumes dissolved oxygen, triggering hypoxic conditions detrimental to aquatic life. In contrast, alkalinization refers to an increase in water pH, often caused by chemical reactions such as carbonate dissolution or anthropogenic inputs, which can alter nutrient availability but is distinct from the nutrient-driven mechanisms underlying eutrophication.
Mechanisms and Drivers of Water Alkalinization
Water alkalinization primarily occurs through enhanced weathering of carbonate and silicate minerals, which increases bicarbonate and carbonate ion concentrations, raising pH levels in aquatic systems. Industrial activities, such as cement production and mining, alongside increased atmospheric CO2 dissolution, accelerate these geochemical processes, driving the alkalinization of water bodies. This mechanism contrasts with eutrophication, which is driven by nutrient enrichment, mainly nitrogen and phosphorus inputs, leading to excessive algal growth rather than chemical changes in water alkalinity.
Ecosystem Impacts: Eutrophication vs Alkalinization
Eutrophication triggers excessive nutrient loading, leading to algal blooms, oxygen depletion, and loss of aquatic biodiversity, severely disrupting freshwater and marine ecosystems. Alkalinization alters water pH by increasing alkalinity, which can impair species sensitive to pH fluctuations and modify nutrient availability, affecting overall ecosystem stability. Both processes shift ecological balance, but eutrophication primarily reduces oxygen levels causing hypoxic zones, whereas alkalinization influences chemical equilibrium, impacting biotic composition and nutrient cycling.
Human Health and Economic Implications
Eutrophication, caused by nutrient pollution, leads to harmful algal blooms that produce toxins affecting human respiratory and neurological health, while requiring costly water treatment and healthcare interventions. Alkalinization, often induced by industrial discharge or excessive use of alkaline substances, disrupts water pH balance, impairing aquatic life and agriculture productivity, which can result in economic losses in fisheries and crop yields. Both processes exacerbate ecosystem degradation, increasing public health risks and imposing significant financial burdens on local economies dependent on natural resources.
Prevention and Management Strategies
Eutrophication prevention involves reducing nutrient inputs, particularly nitrogen and phosphorus, through improved agricultural practices, wastewater treatment, and controlled fertilizer application to limit algal blooms and oxygen depletion. Alkalinization management requires monitoring pH levels and implementing buffering techniques, such as adding acidifying agents or enhancing carbon dioxide exchange, to maintain ecological balance in aquatic systems. Effective strategies combine continuous water quality assessment with ecosystem restoration to mitigate the adverse impacts of both eutrophication and alkalinization.
Future Trends and Research Directions
Future trends in eutrophication research emphasize advanced nutrient management techniques and predictive modeling to mitigate harmful algal blooms, driven by climate change and increasing agricultural runoff. Alkalinization studies focus on enhancing carbon sequestration through ocean alkalinity enhancement, exploring scalable methods to counteract ocean acidification and improve marine ecosystem resilience. Interdisciplinary approaches integrating biogeochemical cycles and environmental policy development are critical for addressing the dual challenges of eutrophication and alkalinization in aquatic systems.
Eutrophication Infographic
 libterm.com
libterm.com