Condensation occurs when water vapor in the air cools and changes into liquid droplets, often forming on surfaces like windows and plants. This process plays a crucial role in the water cycle and influences indoor humidity and comfort levels. Explore the rest of the article to understand how condensation affects your environment and ways to manage it effectively.
Table of Comparison
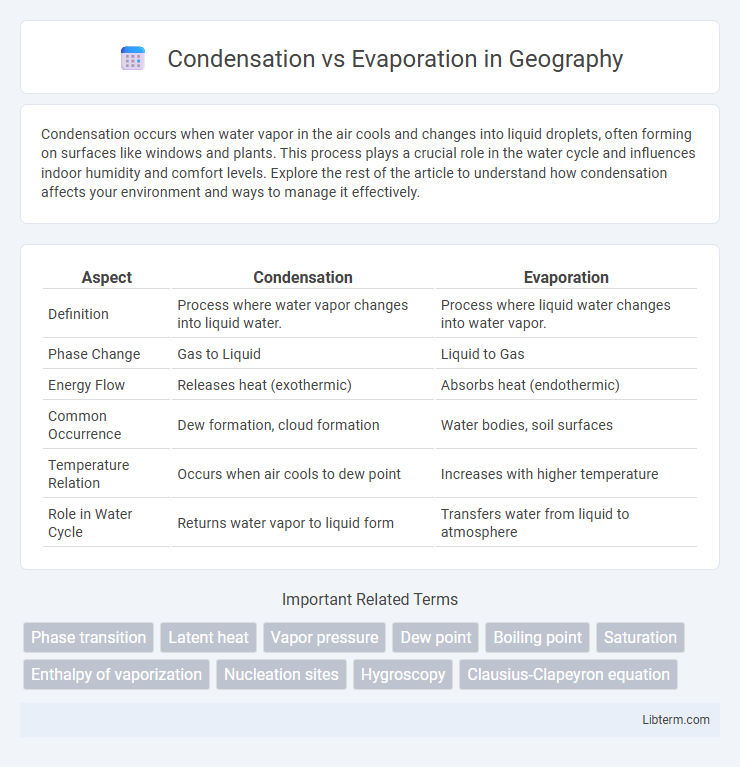
| Aspect | Condensation | Evaporation |
|---|---|---|
| Definition | Process where water vapor changes into liquid water. | Process where liquid water changes into water vapor. |
| Phase Change | Gas to Liquid | Liquid to Gas |
| Energy Flow | Releases heat (exothermic) | Absorbs heat (endothermic) |
| Common Occurrence | Dew formation, cloud formation | Water bodies, soil surfaces |
| Temperature Relation | Occurs when air cools to dew point | Increases with higher temperature |
| Role in Water Cycle | Returns water vapor to liquid form | Transfers water from liquid to atmosphere |
Understanding Condensation and Evaporation
Condensation occurs when water vapor in the air cools and changes into liquid droplets, releasing latent heat and forming clouds, dew, or fog. Evaporation is the process where liquid water absorbs heat energy, transforming into water vapor and entering the atmosphere, playing a crucial role in the water cycle. Understanding these phase changes is essential for studying weather patterns, humidity levels, and climate dynamics.
Key Differences Between Condensation and Evaporation
Condensation is the process where water vapor changes into liquid, typically occurring when air cools and reaches its dew point, whereas evaporation involves the transformation of liquid water into vapor due to heat energy. Condensation releases latent heat back into the environment, while evaporation absorbs heat, cooling the surrounding area. Key differences also include the phase change direction--condensation is gas to liquid, evaporation is liquid to gas--and the typical environmental conditions favoring each, with condensation common in cooler, saturated air and evaporation prevalent in warmer, unsaturated conditions.
The Science Behind Condensation
Condensation occurs when water vapor in the air cools down and changes from a gas to a liquid state, typically forming droplets on surfaces. This phase change happens because cooler temperatures reduce the kinetic energy of water molecules, causing them to cluster together. The science behind condensation is crucial in understanding natural processes like cloud formation, dew, and the water cycle.
How Evaporation Occurs
Evaporation occurs when molecules at the surface of a liquid gain enough energy to overcome intermolecular forces and transition into the gas phase. This process is influenced by factors such as temperature, surface area, humidity, and air movement, which increase the rate at which liquid molecules escape into the atmosphere. Evaporation plays a critical role in the water cycle by transferring water from oceans, lakes, and soil into the air as water vapor.
Factors Influencing Condensation
Temperature plays a critical role in condensation, with cooler surfaces facilitating the transformation of water vapor into liquid droplets. Humidity levels directly impact condensation rates, as higher moisture content increases the likelihood of vapor turning into liquid. Air pressure and the presence of condensation nuclei such as dust particles also significantly influence the formation and efficiency of condensation processes.
Factors Affecting Evaporation Rates
Evaporation rates are primarily influenced by temperature, surface area, humidity, and wind speed. Higher temperatures increase molecular energy, accelerating evaporation, while larger surface areas provide more space for water molecules to escape. Low humidity and strong airflow both enhance evaporation by reducing water vapor concentration near the liquid surface, facilitating faster moisture loss.
Real-World Examples of Condensation
Condensation occurs when water vapor cools and changes into liquid droplets, commonly seen as dew forming on grass in the early morning or water droplets accumulating on the outside of a cold beverage glass. This process plays a critical role in the water cycle by facilitating cloud formation, which eventually leads to precipitation. In everyday life, condensation is also observed in bathroom mirrors fogging up during a hot shower and on cold windows during winter as warm indoor air meets the cold glass surface.
Everyday Examples of Evaporation
Evaporation occurs when water transforms from a liquid to a gas, a process commonly seen when puddles dry up after rain or when sweat evaporates from the skin to cool the body. The heat energy from sunlight or air increases the kinetic energy of water molecules, allowing them to escape into the atmosphere. Household examples include clothes drying on a line and water boiling in a kettle, showcasing evaporation's role in everyday moisture regulation.
Importance of Condensation and Evaporation in Nature
Condensation plays a crucial role in the water cycle by transforming water vapor into liquid, leading to the formation of clouds and precipitation that sustain ecosystems and replenish freshwater sources. Evaporation drives the transfer of moisture from terrestrial and aquatic surfaces into the atmosphere, regulating temperature and enabling nutrient cycling essential for plant growth and climate balance. Together, condensation and evaporation maintain atmospheric moisture levels, support biodiversity, and influence global weather patterns.
Practical Applications and Impacts
Condensation is vital in HVAC systems for moisture removal and improving indoor air quality, while evaporation drives cooling processes in natural and industrial settings, such as in cooling towers and evaporative coolers. Both processes are crucial in water cycle management, influencing agricultural irrigation through soil moisture retention (condensation) and crop transpiration (evaporation). Understanding these phase changes helps optimize energy efficiency, control humidity, and support environmental sustainability in engineering and environmental science applications.
Condensation Infographic
 libterm.com
libterm.com