Acidification disrupts natural ecosystems by lowering the pH levels of oceans and soils, harming marine life and plant growth. Increased carbon dioxide emissions accelerate this process, leading to widespread environmental consequences. Discover how acidification impacts your world and what measures can help combat its effects in the following article.
Table of Comparison
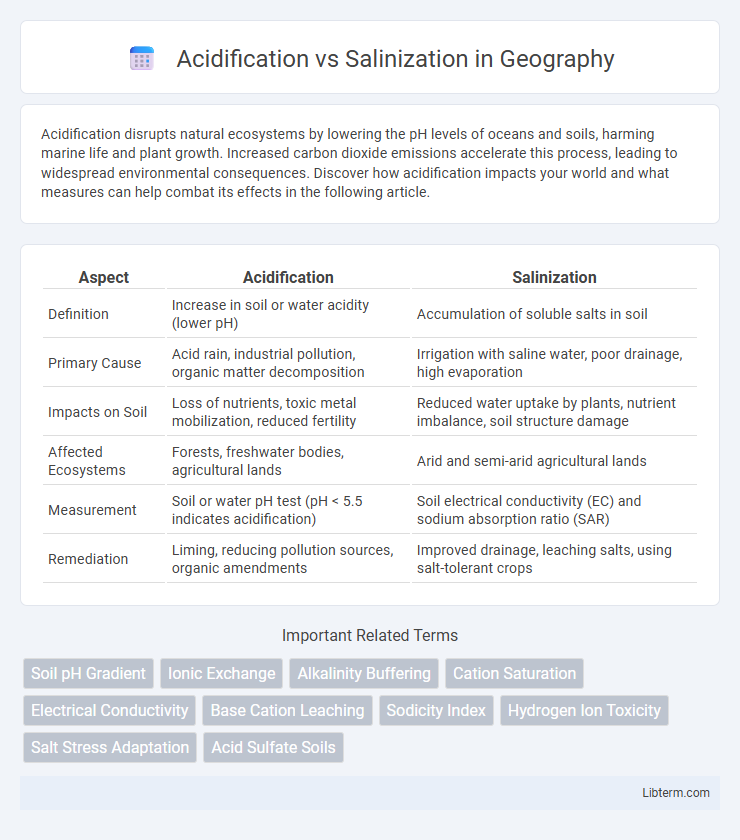
| Aspect | Acidification | Salinization |
|---|---|---|
| Definition | Increase in soil or water acidity (lower pH) | Accumulation of soluble salts in soil |
| Primary Cause | Acid rain, industrial pollution, organic matter decomposition | Irrigation with saline water, poor drainage, high evaporation |
| Impacts on Soil | Loss of nutrients, toxic metal mobilization, reduced fertility | Reduced water uptake by plants, nutrient imbalance, soil structure damage |
| Affected Ecosystems | Forests, freshwater bodies, agricultural lands | Arid and semi-arid agricultural lands |
| Measurement | Soil or water pH test (pH < 5.5 indicates acidification) | Soil electrical conductivity (EC) and sodium absorption ratio (SAR) |
| Remediation | Liming, reducing pollution sources, organic amendments | Improved drainage, leaching salts, using salt-tolerant crops |
Understanding Acidification and Salinization
Acidification refers to the process by which soils or water bodies become more acidic due to increased hydrogen ion concentration, often caused by industrial emissions or acid rain. Salinization occurs when soluble salts accumulate in the soil, reducing its fertility and affecting plant growth, commonly resulting from irrigation with saline water or poor drainage. Understanding these processes is critical for managing soil health and preventing crop yield decline in agricultural ecosystems.
Key Causes of Acidification in Ecosystems
Acidification in ecosystems primarily results from the deposition of sulfur and nitrogen oxides emitted by fossil fuel combustion, leading to acid rain that lowers soil and water pH levels. Industrial activities, mining, and agricultural runoff also contribute to increased acidity by releasing acidic compounds and heavy metals into the environment. These changes disrupt nutrient availability and harm aquatic life, soil microorganisms, and vegetation.
Primary Drivers of Salinization Processes
Primary drivers of salinization processes include irrigation with saline water, inadequate drainage, and high evaporation rates, which lead to the accumulation of soluble salts in soil. Excessive use of fertilizers and industrial discharges also contribute to increased soil salinity by altering the natural balance of ions. These factors disrupt soil structure and reduce agricultural productivity, distinguishing salinization from acidification, which is primarily driven by acid rain and chemical imbalances.
Chemical Mechanisms: Acidification vs Salinization
Acidification occurs when soil or water pH decreases due to the influx of hydrogen ions (H+), often from acid rain or the oxidation of sulfur and nitrogen compounds, leading to the mobilization of toxic metals like aluminum. Salinization involves the accumulation of soluble salts, primarily sodium chloride (NaCl), in soil caused by irrigation with saline water or poor drainage, resulting in osmotic stress and ion toxicity to plants. Both processes disrupt soil chemical equilibrium but differ in ion composition; acidification increases H+ concentration, while salinization elevates salt ions, affecting nutrient availability and microbial activity distinctly.
Impact on Soil Health and Fertility
Acidification reduces soil pH, leading to nutrient imbalances and toxic aluminum availability, which inhibits microbial activity and root growth, ultimately decreasing soil fertility. Salinization accumulates soluble salts in the soil, causing osmotic stress that restricts plant water uptake and disrupts microbial processes, resulting in reduced soil productivity. Both processes degrade soil structure and nutrient cycling, severely impacting long-term soil health and crop yields.
Effects on Aquatic Ecosystems and Water Quality
Acidification lowers water pH, leading to toxic conditions for aquatic life, disrupting reproductive cycles and reducing biodiversity, while increasing metal solubility harms fish and invertebrates. Salinization raises salt concentrations, causing osmotic stress that impairs freshwater species' survival and alters species composition, often reducing freshwater biodiversity. Both processes degrade water quality by altering chemical balances, increasing pollutant bioavailability, and threatening ecosystem stability.
Consequences for Agricultural Productivity
Acidification reduces soil pH, leading to nutrient imbalances and toxic aluminum availability, which impair root development and decrease crop yields. Salinization causes high salt concentrations that hinder water uptake by plant roots, resulting in osmotic stress and reduced growth. Both processes significantly diminish agricultural productivity by compromising soil health and plant nutrient absorption.
Mitigation Strategies for Acidification
Mitigation strategies for acidification primarily involve reducing emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) through the adoption of cleaner energy sources and implementation of stricter air quality regulations. Soil liming with calcium carbonate is widely used to neutralize acidic soils and restore pH balance, improving nutrient availability and microbial activity. Restoration of vegetation cover enhances natural buffering capacity and reduces acid deposition impacts in ecosystems affected by acid rain.
Managing and Preventing Salinization
Managing and preventing salinization involves improving irrigation practices by using efficient drainage systems and avoiding over-irrigation to reduce salt buildup in soil. Selecting salt-tolerant crops and applying soil amendments such as gypsum help maintain soil structure and reduce salinity stress. Regular monitoring of soil salinity levels enables early detection, allowing timely interventions to protect agricultural productivity and soil health.
Future Challenges and Sustainable Solutions
Future challenges in acidification and salinization include worsening soil degradation, reduced agricultural productivity, and increased vulnerability to climate change impacts. Sustainable solutions emphasize implementing precision agriculture, enhancing soil organic matter through cover cropping and biochar application, and adopting integrated water management systems to mitigate salt buildup and acidification. Developing salt- and acid-tolerant crop varieties through biotechnology offers promising resilience against these soil threats.
Acidification Infographic
 libterm.com
libterm.com