Bioequivalence ensures that two drug products release their active ingredients into the bloodstream at the same rate and extent, directly impacting therapeutic effectiveness. Clinical equivalence guarantees that these products provide comparable safety and efficacy outcomes in patients, making them interchangeable for treatment purposes. Explore the rest of the article to understand how these concepts affect Your medication choices and regulatory approvals.
Table of Comparison
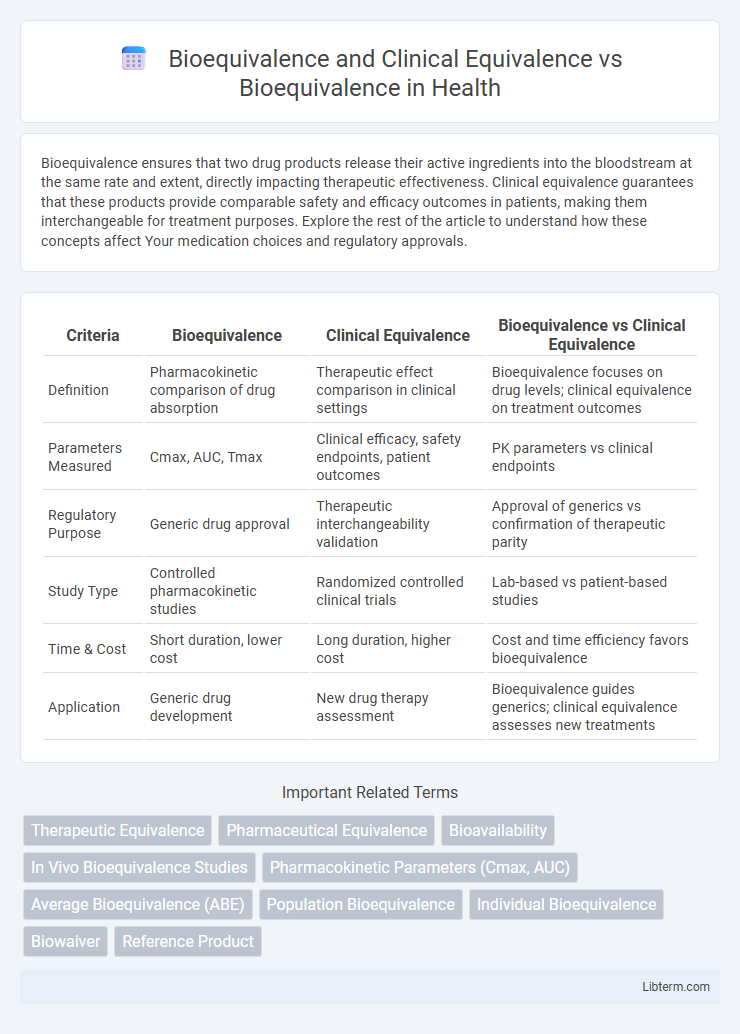
| Criteria | Bioequivalence | Clinical Equivalence | Bioequivalence vs Clinical Equivalence |
|---|---|---|---|
| Definition | Pharmacokinetic comparison of drug absorption | Therapeutic effect comparison in clinical settings | Bioequivalence focuses on drug levels; clinical equivalence on treatment outcomes |
| Parameters Measured | Cmax, AUC, Tmax | Clinical efficacy, safety endpoints, patient outcomes | PK parameters vs clinical endpoints |
| Regulatory Purpose | Generic drug approval | Therapeutic interchangeability validation | Approval of generics vs confirmation of therapeutic parity |
| Study Type | Controlled pharmacokinetic studies | Randomized controlled clinical trials | Lab-based vs patient-based studies |
| Time & Cost | Short duration, lower cost | Long duration, higher cost | Cost and time efficiency favors bioequivalence |
| Application | Generic drug development | New drug therapy assessment | Bioequivalence guides generics; clinical equivalence assesses new treatments |
Introduction to Bioequivalence
Bioequivalence refers to the pharmacokinetic equivalence between two drug formulations, ensuring they have similar bioavailability and produce identical concentration-time profiles in plasma. Clinical equivalence extends beyond bioequivalence by demonstrating therapeutic equivalence through clinical trials, confirming similar efficacy and safety in target patient populations. Introduction to Bioequivalence highlights its critical role in generic drug approval, reducing the need for extensive clinical testing by proving comparable bioavailability to the reference innovator product.
Understanding Clinical Equivalence
Clinical equivalence demonstrates that two treatments provide the same therapeutic effect and safety profile in real-world patient care, while bioequivalence specifically measures that two drug formulations release the active ingredient into the bloodstream at the same rate and extent. Understanding clinical equivalence involves assessing outcomes such as efficacy, safety, and patient-reported results across diverse populations, going beyond pharmacokinetic parameters. Regulatory approval relies on clinical equivalence data to ensure that generic or alternative therapies deliver comparable clinical benefits to the reference product.
Bioequivalence vs. Clinical Equivalence: Key Differences
Bioequivalence refers to the comparison of the same active ingredient in different formulations, ensuring similar bioavailability and pharmacokinetic properties, typically assessed through parameters like Cmax and AUC. Clinical equivalence goes beyond pharmacokinetics to demonstrate that two drugs have comparable therapeutic effects and safety profiles in patients, often requiring extensive clinical trials. The key difference lies in bioequivalence focusing on drug absorption and systemic exposure, while clinical equivalence emphasizes actual clinical outcomes and therapeutic interchangeability.
Regulatory Standards for Bioequivalence
Regulatory standards for bioequivalence require demonstrating that the generic drug's rate and extent of absorption do not show a significant difference from the reference drug, typically within a 90% confidence interval of 80-125% for key pharmacokinetic parameters like Cmax and AUC. Clinical equivalence focuses on comparable therapeutic outcomes, but bioequivalence emphasizes pharmacokinetic measures as a proxy to infer similar clinical performance, streamlining regulatory approval without extensive clinical trials. Agencies such as the FDA and EMA enforce these bioequivalence criteria to ensure generic drugs provide the same safety and efficacy as their branded counterparts.
Methods for Assessing Bioequivalence
Methods for assessing bioequivalence primarily involve pharmacokinetic studies that measure drug concentration in plasma over time, focusing on parameters such as Cmax (maximum concentration) and AUC (area under the curve) to ensure comparable drug absorption rates and extents between test and reference products. Clinical equivalence differs by emphasizing direct clinical outcome comparisons, often requiring therapeutic equivalence trials to demonstrate similar efficacy and safety profiles. The gold standard for bioequivalence assessment remains crossover design studies using statistical analyses like 90% confidence intervals within predefined bioequivalence limits (80-125%) for key pharmacokinetic metrics.
Clinical Equivalence: Assessment Criteria
Clinical equivalence assessment criteria emphasize therapeutic effect similarity, safety profiles, and pharmacodynamic outcomes to confirm that two medicinal products are interchangeable without significant differences in clinical outcomes. Unlike bioequivalence, which primarily relies on pharmacokinetic metrics such as Cmax and AUC to compare drug absorption and systemic exposure, clinical equivalence requires robust clinical trial data demonstrating no meaningful difference in efficacy or adverse events. Regulatory guidelines often mandate double-blind, randomized controlled trials to ensure rigorous evaluation of clinical equivalence beyond bioequivalence parameters.
Importance of Bioequivalence in Generic Drug Approval
Bioequivalence is crucial in generic drug approval as it ensures that the generic product releases the same active ingredient into the bloodstream at the same rate and extent as the original branded drug, thereby guaranteeing therapeutic equivalence. Unlike clinical equivalence, which involves extensive clinical trials to demonstrate similar efficacy and safety, bioequivalence relies on pharmacokinetic studies to confirm that the generic drug performs similarly without duplicative clinical testing. Regulatory agencies prioritize bioequivalence to streamline generic approvals, reduce costs, and increase access to affordable medications while maintaining drug safety and effectiveness.
Challenges in Demonstrating Clinical Equivalence
Demonstrating clinical equivalence poses significant challenges due to variability in patient responses, disease progression, and study design complexities that are not as prominent in bioequivalence studies focused on pharmacokinetic parameters. Clinical equivalence requires comprehensive efficacy and safety data from well-controlled clinical trials, which are often time-consuming and costly compared to the more straightforward bioequivalence assessment based on plasma concentration metrics. Interindividual differences and ethical considerations further complicate the demonstration of clinical equivalence, making it critical to carefully select endpoints and patient populations to achieve robust and reproducible outcomes.
Case Studies: Bioequivalence and Clinical Equivalence in Practice
Case studies in bioequivalence and clinical equivalence demonstrate the practical evaluation of generic drugs against brand-name counterparts by comparing pharmacokinetic parameters such as Cmax and AUC to ensure similar bioavailability. Clinical equivalence assessments extend beyond bioequivalence by incorporating therapeutic outcomes, adverse effects, and patient-reported measures to confirm that two treatments provide comparable clinical benefits and safety profiles. Examples from oncology and cardiovascular drug trials highlight challenges in establishing clinical equivalence despite bioequivalence, underscoring the importance of comprehensive clinical evaluations in regulatory approvals.
Future Perspectives in Bioequivalence and Clinical Equivalence
Future perspectives in bioequivalence emphasize integrating advanced in vitro and in silico models to predict pharmacokinetic profiles, enhancing the precision of generic drug approval processes. Innovations in clinical equivalence assessments aim to incorporate real-world evidence and biomarker-driven endpoints to better capture therapeutic outcomes beyond pharmacokinetics. Regulatory frameworks are evolving to support adaptive trial designs and artificial intelligence applications, streamlining bioequivalence evaluations while ensuring patient safety and efficacy.
Bioequivalence and Clinical Equivalence Infographic
 libterm.com
libterm.com