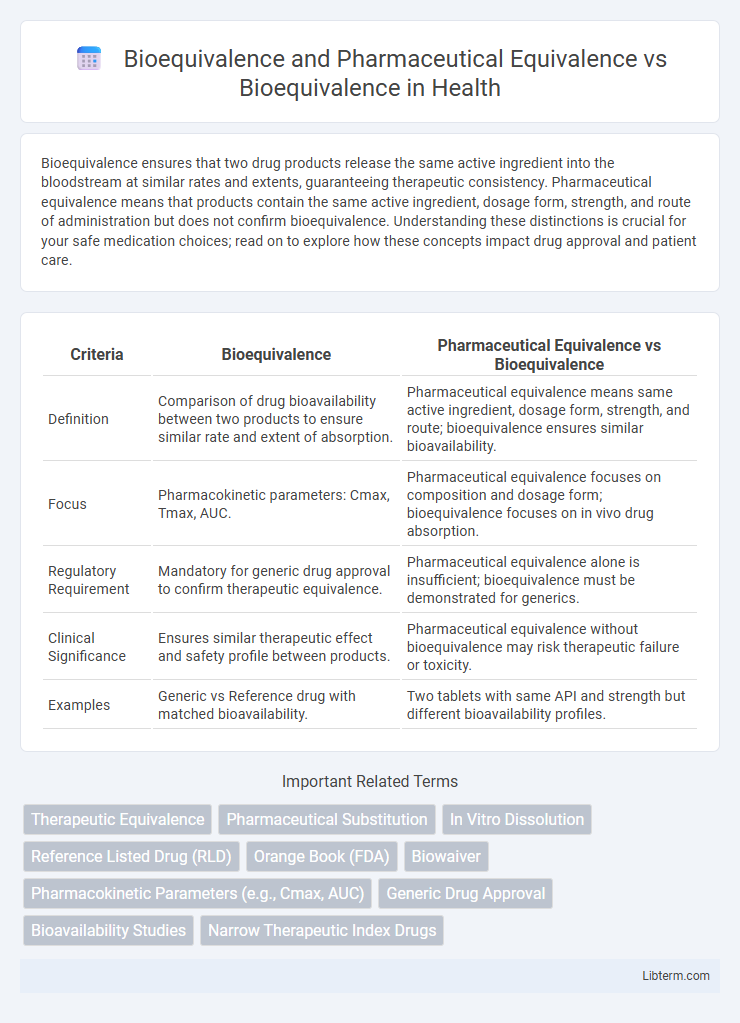
Bioequivalence ensures that two drug products release the same active ingredient into the bloodstream at similar rates and extents, guaranteeing therapeutic consistency. Pharmaceutical equivalence means that products contain the same active ingredient, dosage form, strength, and route of administration but does not confirm bioequivalence. Understanding these distinctions is crucial for your safe medication choices; read on to explore how these concepts impact drug approval and patient care.
Table of Comparison
| Criteria | Bioequivalence | Pharmaceutical Equivalence vs Bioequivalence |
|---|---|---|
| Definition | Comparison of drug bioavailability between two products to ensure similar rate and extent of absorption. | Pharmaceutical equivalence means same active ingredient, dosage form, strength, and route; bioequivalence ensures similar bioavailability. |
| Focus | Pharmacokinetic parameters: Cmax, Tmax, AUC. | Pharmaceutical equivalence focuses on composition and dosage form; bioequivalence focuses on in vivo drug absorption. |
| Regulatory Requirement | Mandatory for generic drug approval to confirm therapeutic equivalence. | Pharmaceutical equivalence alone is insufficient; bioequivalence must be demonstrated for generics. |
| Clinical Significance | Ensures similar therapeutic effect and safety profile between products. | Pharmaceutical equivalence without bioequivalence may risk therapeutic failure or toxicity. |
| Examples | Generic vs Reference drug with matched bioavailability. | Two tablets with same API and strength but different bioavailability profiles. |
Introduction to Bioequivalence and Pharmaceutical Equivalence
Bioequivalence refers to the absence of a significant difference in the rate and extent of absorption of the active pharmaceutical ingredient between a test drug and its reference product under similar conditions. Pharmaceutical equivalence means that drug products contain the same active ingredient(s), dosage form, strength, and route of administration but may differ in characteristics such as shape, scoring, release mechanism, or packaging. Understanding bioequivalence is essential for ensuring therapeutic equivalence, while pharmaceutical equivalence confirms that drug products are identical in their qualitative and quantitative composition.
Defining Bioequivalence: Key Concepts
Bioequivalence refers to the absence of a significant difference in the bioavailability of two pharmaceutical products containing the same active ingredient, ensuring they deliver the same therapeutic effect. Pharmaceutical equivalence means drug products contain identical amounts of the same active ingredient in the same dosage form and strength but does not guarantee bioequivalence or therapeutic equivalence. Key concepts of bioequivalence focus on pharmacokinetic parameters such as Cmax (maximum concentration) and AUC (area under the curve), demonstrating similar rate and extent of absorption between the test and reference products.
Understanding Pharmaceutical Equivalence
Pharmaceutical equivalence refers to drug products that contain the same active ingredient(s), dosage form, strength, and route of administration, ensuring consistency in formulation. This concept is crucial for regulatory approval as it confirms that the generic drug matches the brand-name drug in essential characteristics, except for non-active components. Understanding pharmaceutical equivalence is foundational before assessing bioequivalence, which measures the rate and extent of absorption of the active ingredient, confirming therapeutic equivalence.
Regulatory Perspectives on Bioequivalence and Pharmaceutical Equivalence
Regulatory perspectives on bioequivalence emphasize demonstrating that a generic product releases its active ingredient into the bloodstream at the same rate and extent as the reference drug, ensuring therapeutic equivalence. Pharmaceutical equivalence requires that products contain the same active ingredient, dosage form, strength, and route of administration, but does not guarantee similar bioavailability or clinical performance. Regulatory agencies such as the FDA mandate both pharmaceutical equivalence and bioequivalence testing to approve generic drugs, ensuring safety, efficacy, and interchangeability with branded products.
Bioequivalence vs Pharmaceutical Equivalence: Fundamental Differences
Bioequivalence refers to the absence of significant difference in the rate and extent to which the active pharmaceutical ingredient becomes available at the site of drug action when administered at the same molar dose under similar conditions. Pharmaceutical equivalence means that drug products contain identical amounts of the same active ingredient, dosage form, strength, and route of administration but does not guarantee similar bioavailability or therapeutic effect. The fundamental difference lies in bioequivalence assessing in vivo performance and therapeutic equivalence, while pharmaceutical equivalence solely assures formulation similarity without confirming clinical interchangeability.
Importance of Bioequivalence in Drug Approval
Bioequivalence ensures that a generic drug releases its active ingredient into the bloodstream at the same rate and extent as the brand-name counterpart, which is critical for therapeutic consistency. Pharmaceutical equivalence guarantees that two drugs contain the same active ingredients in identical dosage forms and strengths but does not account for their bioavailability. Regulatory agencies prioritize bioequivalence studies during drug approval to confirm that generics are safe, effective, and therapeutically interchangeable with innovator drugs.
Methods for Assessing Bioequivalence
Methods for assessing bioequivalence typically involve pharmacokinetic studies measuring parameters such as Cmax (maximum concentration), Tmax (time to reach Cmax), and AUC (area under the curve) to compare a generic drug with its reference product. Pharmaceutical equivalence ensures two drugs contain the same active ingredient in the same dosage form and strength, whereas bioequivalence demonstrates comparable bioavailability and therapeutic effect through clinical and laboratory testing. Regulatory agencies like the FDA require rigorous crossover study designs and statistical analysis of bioequivalence data to confirm that variations between products do not affect efficacy or safety.
Case Studies: Bioequivalence in Generic Drugs
Case studies on bioequivalence in generic drugs demonstrate that pharmaceutical equivalence ensures identical active ingredients and dosage forms, while bioequivalence verifies similar bioavailability and pharmacokinetic profiles compared to brand-name drugs. Regulatory submissions for generic approvals require rigorous in vivo bioequivalence trials, often using crossover designs to confirm therapeutic equivalence. Evidence from FDA case studies highlights that minor variations in excipients do not compromise drug safety or efficacy, reinforcing bioequivalence as a critical standard for generic drug approval.
Challenges in Establishing Bioequivalence and Pharmaceutical Equivalence
Establishing bioequivalence involves rigorous in vivo studies to demonstrate comparable pharmacokinetic profiles, whereas pharmaceutical equivalence primarily requires identical active ingredients, dosage forms, and strength. Challenges in bioequivalence include variability in drug absorption, metabolism differences among populations, and the complexity of measuring bioavailability for extended-release formulations. Pharmaceutical equivalence faces difficulties in ensuring consistent excipient composition and manufacturing processes that do not impact drug release and therapeutic efficacy.
Future Trends in Bioequivalence Research
Future trends in bioequivalence research emphasize advanced modeling techniques, such as physiologically-based pharmacokinetic (PBPK) modeling, to predict drug absorption and enhance regulatory decision-making. The integration of artificial intelligence and machine learning algorithms aims to improve the accuracy and efficiency of bioequivalence assessments, reducing reliance on costly and time-consuming clinical studies. Emerging approaches also focus on personalized medicine, considering genetic and demographic factors to refine bioequivalence criteria for diverse patient populations.
Bioequivalence and Pharmaceutical Equivalence Infographic
 libterm.com
libterm.com