Pharmacoepidemiology studies the use and effects of medications in large populations, combining principles from pharmacology and epidemiology to understand drug safety, efficacy, and patterns of use. This field plays a crucial role in identifying adverse drug reactions and informing public health policies to optimize medication therapy. Explore the rest of this article to discover how pharmacoepidemiology impacts your healthcare decisions and medication management.
Table of Comparison
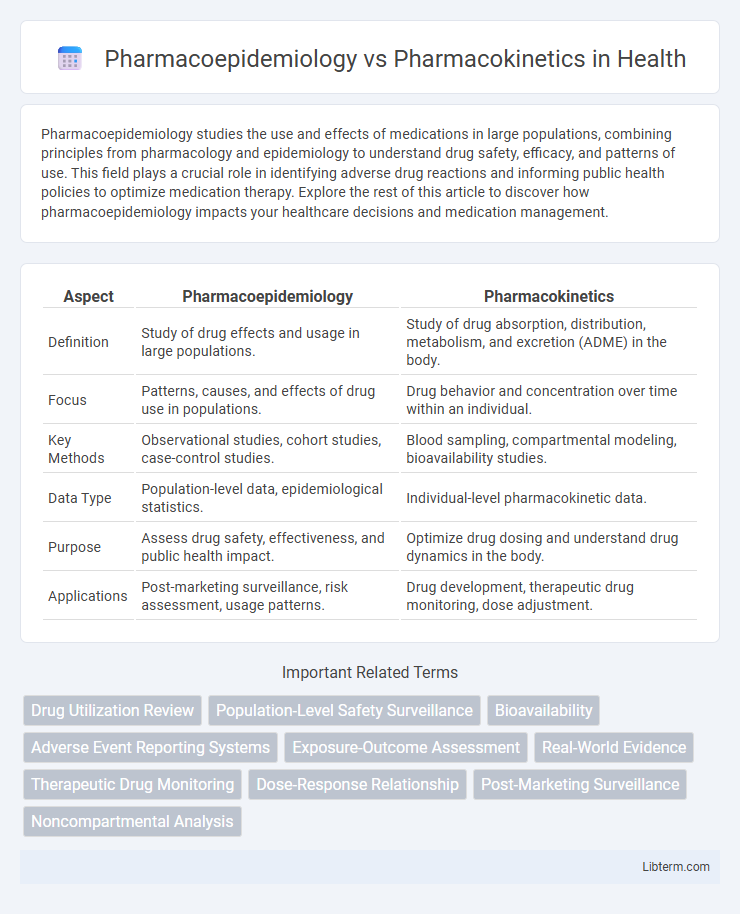
| Aspect | Pharmacoepidemiology | Pharmacokinetics |
|---|---|---|
| Definition | Study of drug effects and usage in large populations. | Study of drug absorption, distribution, metabolism, and excretion (ADME) in the body. |
| Focus | Patterns, causes, and effects of drug use in populations. | Drug behavior and concentration over time within an individual. |
| Key Methods | Observational studies, cohort studies, case-control studies. | Blood sampling, compartmental modeling, bioavailability studies. |
| Data Type | Population-level data, epidemiological statistics. | Individual-level pharmacokinetic data. |
| Purpose | Assess drug safety, effectiveness, and public health impact. | Optimize drug dosing and understand drug dynamics in the body. |
| Applications | Post-marketing surveillance, risk assessment, usage patterns. | Drug development, therapeutic drug monitoring, dose adjustment. |
Introduction to Pharmacoepidemiology and Pharmacokinetics
Pharmacoepidemiology studies the distribution and determinants of drug effects in large populations to optimize medication use and ensure drug safety. Pharmacokinetics examines the body's absorption, distribution, metabolism, and excretion (ADME) processes to determine drug concentration profiles over time. Both fields provide critical insights for drug development, regulatory decisions, and personalized medicine by integrating population-level outcomes with individual drug behavior.
Definitions and Core Concepts
Pharmacoepidemiology studies the use and effects of drugs in large populations, focusing on patterns, causes, and outcomes of medication use to assess safety and effectiveness in real-world settings. Pharmacokinetics examines the absorption, distribution, metabolism, and excretion (ADME) of drugs within an individual, detailing how the body processes a drug over time. While pharmacoepidemiology provides data on drug impact at the population level, pharmacokinetics offers insights into the drug's behavior at the molecular and systemic level in the body.
Scope and Applications in Drug Research
Pharmacoepidemiology focuses on studying the use and effects of drugs in large populations, emphasizing drug safety, effectiveness, and patterns of drug utilization in clinical and public health settings. Pharmacokinetics examines the absorption, distribution, metabolism, and excretion (ADME) of drugs within an individual, highlighting dose-response relationships and optimizing drug dosing regimens. Both disciplines are critical in drug research, with pharmacoepidemiology guiding post-marketing surveillance and real-world evidence generation, while pharmacokinetics informs clinical trial design and personalized medicine strategies.
Key Methodologies and Study Designs
Pharmacoepidemiology employs large-scale observational studies and database analyses to assess drug effects in populations, utilizing cohort, case-control, and cross-sectional study designs. Pharmacokinetics focuses on experimental methods such as compartmental modeling, non-compartmental analysis, and bioavailability studies to measure drug absorption, distribution, metabolism, and excretion in individuals. Both fields rely on robust statistical modeling, but pharmacoepidemiology emphasizes real-world evidence while pharmacokinetics prioritizes controlled clinical or laboratory settings.
Main Objectives: Population vs. Individual Focus
Pharmacoepidemiology primarily investigates drug effects and usage patterns within large populations to understand safety, efficacy, and risk factors on a broad scale. Pharmacokinetics focuses on the individual by studying the absorption, distribution, metabolism, and excretion of drugs to optimize personalized dosing and therapeutic outcomes. Both disciplines complement each other by bridging population-level drug utilization data with individual patient-specific pharmacological responses.
Data Sources and Analysis Techniques
Pharmacoepidemiology primarily utilizes large healthcare databases, electronic health records, and insurance claims data to study drug effects across populations, employing statistical techniques such as cohort studies, case-control studies, and regression analysis. Pharmacokinetics relies on clinical trial data and bioanalytical measurements, analyzing drug absorption, distribution, metabolism, and excretion using compartmental modeling and non-compartmental analysis methods. Both fields leverage advanced software tools for data processing, but pharmacoepidemiology emphasizes real-world evidence while pharmacokinetics focuses on controlled experimental data.
Impact on Drug Safety and Efficacy Assessments
Pharmacoepidemiology analyzes drug effects and safety in large populations, providing real-world data on adverse reactions and long-term efficacy that inform regulatory decisions and post-marketing surveillance. Pharmacokinetics studies drug absorption, distribution, metabolism, and excretion at the individual level, optimizing dosing regimens to enhance therapeutic outcomes and minimize toxicity. Integrating pharmacoepidemiological data with pharmacokinetic profiles improves drug safety assessments and efficacy predictions by combining population trends with detailed mechanistic insights.
Role in Public Health and Clinical Practice
Pharmacoepidemiology investigates the use and effects of medications in large populations to identify patterns, risks, and benefits, playing a crucial role in public health by informing drug safety and policy decisions. Pharmacokinetics analyzes the absorption, distribution, metabolism, and excretion of drugs within individual patients, guiding clinical practice through dosage optimization and therapeutic monitoring. Together, these disciplines complement each other by bridging population-level drug impact with personalized treatment strategies.
Challenges and Limitations of Each Field
Pharmacoepidemiology faces challenges in controlling confounding variables and biases inherent in observational data, limiting the accuracy of causal inferences about drug effects in populations. Pharmacokinetics encounters limitations in translating in vitro and animal model data to human metabolism, complicated by individual variability in absorption, distribution, metabolism, and excretion. Both fields require integration of complex datasets and advanced statistical models to overcome these obstacles and improve drug safety and efficacy evaluations.
Integrating Pharmacoepidemiology and Pharmacokinetics for Improved Outcomes
Integrating pharmacoepidemiology and pharmacokinetics enhances medication safety and efficacy by combining real-world drug utilization data with individual metabolic profiles, leading to personalized therapy strategies. This multidisciplinary approach enables the identification of population-level drug response patterns while accounting for pharmacokinetic variability, improving risk assessment and dosage optimization. Advanced models incorporating pharmacokinetic parameters and epidemiological data support targeted interventions, reducing adverse drug reactions and enhancing therapeutic outcomes across diverse patient populations.
Pharmacoepidemiology Infographic
 libterm.com
libterm.com