Platinum is a rare, durable metal prized for its resistance to tarnish and corrosion, making it ideal for fine jewelry and industrial applications. Its high density and impressive conductivity enhance performance in catalytic converters and electronics. Discover how platinum's unique properties can benefit your needs by exploring the full article.
Table of Comparison
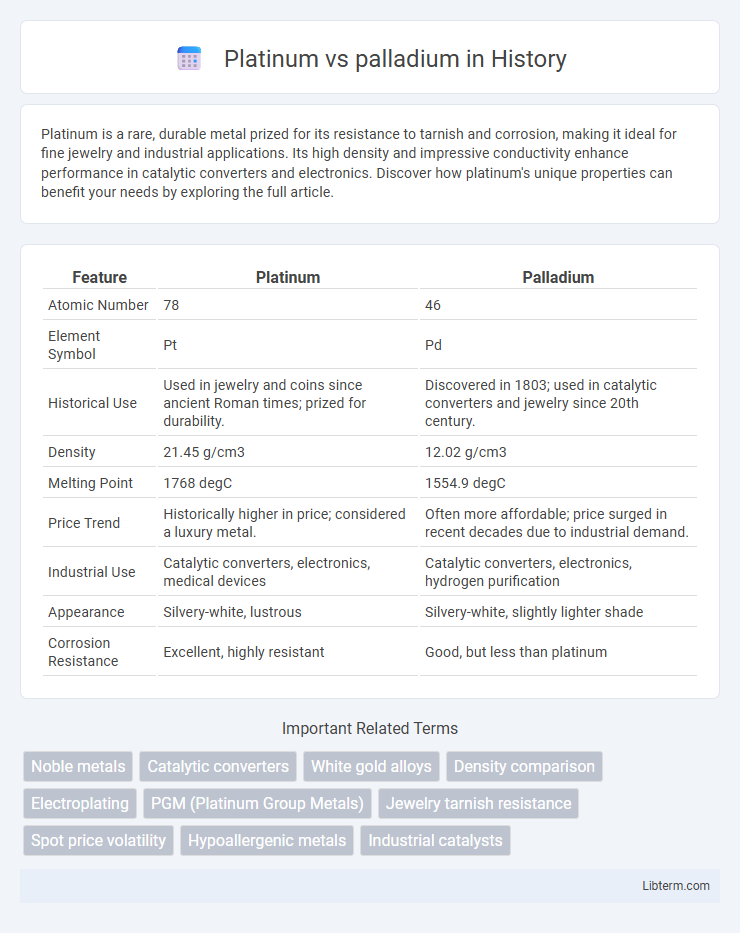
| Feature | Platinum | Palladium |
|---|---|---|
| Atomic Number | 78 | 46 |
| Element Symbol | Pt | Pd |
| Historical Use | Used in jewelry and coins since ancient Roman times; prized for durability. | Discovered in 1803; used in catalytic converters and jewelry since 20th century. |
| Density | 21.45 g/cm3 | 12.02 g/cm3 |
| Melting Point | 1768 degC | 1554.9 degC |
| Price Trend | Historically higher in price; considered a luxury metal. | Often more affordable; price surged in recent decades due to industrial demand. |
| Industrial Use | Catalytic converters, electronics, medical devices | Catalytic converters, electronics, hydrogen purification |
| Appearance | Silvery-white, lustrous | Silvery-white, slightly lighter shade |
| Corrosion Resistance | Excellent, highly resistant | Good, but less than platinum |
Introduction: Platinum vs Palladium Overview
Platinum and palladium are precious metals belonging to the platinum group metals (PGMs), widely used in automotive catalytic converters, jewelry, and industrial applications. Platinum is denser, more corrosion-resistant, and traditionally more expensive, while palladium has gained popularity due to its lighter weight and rising demand in emission control technologies. Market fluctuations and supply constraints influence their pricing, making both metals critical in the global economy and green technology sectors.
Chemical and Physical Properties Comparison
Platinum and palladium are both transition metals in the platinum group, with atomic numbers 78 and 46, respectively, showcasing similar corrosion resistance and catalytic properties. Platinum has a higher melting point of 1,768degC compared to palladium's 1,555degC, and it is denser, weighing 21.45 g/cm3 versus palladium's 12.02 g/cm3. Chemically, platinum is more inert, resisting acids more effectively, while palladium exhibits higher hydrogen absorption capacity, making it critical in hydrogen storage and catalytic converters.
Historical Uses and Discovery
Platinum was first discovered in South America in the early 18th century and historically used by pre-Columbian cultures for ornamental and ceremonial objects due to its rarity and resistance to corrosion. Palladium, discovered in 1803 by William Hyde Wollaston in England, quickly found applications in jewelry and dentistry because of its similar appearance to platinum but lower cost and lighter weight. Both metals have historically played crucial roles in industrial advancements, with platinum widely utilized in catalytic converters and palladium increasingly important in electronics and automotive catalysts.
Applications in Industry and Jewelry
Platinum is widely used in catalytic converters, jewelry, electrical contacts, and medical devices due to its exceptional durability and resistance to corrosion. Palladium finds extensive application in catalytic converters, electronics, hydrogen storage, and as a key component in white gold and platinum jewelry alloys, prized for its lightweight and hypoallergenic properties. Both metals serve critical roles in automotive emissions control and luxury jewelry markets, but palladium's rising demand in electronics contrasts with platinum's higher stability and density preferred in premium jewelry and industrial catalysts.
Market Value and Price Trends
Platinum and palladium are both precious metals with distinct market value trajectories, driven by industrial demand and rarity. Platinum generally trades at a premium due to its higher density and extensive use in catalytic converters for diesel engines, while palladium often sees sharper price increases fueled by its critical role in gasoline vehicle emissions control. Recent trends indicate palladium prices have surged past platinum, reflecting tighter supply and growing demand in automotive and electronics industries.
Rarity and Global Supply Sources
Platinum is rarer than palladium, with annual production around 190 metric tons compared to palladium's approximately 210 metric tons, making supply more constrained. South Africa and Russia dominate global platinum supply, while palladium is primarily sourced from Russia and South Africa, with notable contributions from North America. This disparity in rarity and concentrated geographic supply significantly impacts market dynamics and pricing trends for both metals.
Catalytic Performance in Automotive Industry
Platinum and palladium are both critical catalysts in the automotive industry for reducing harmful emissions, but palladium outperforms platinum in gasoline engine catalytic converters due to its superior oxygen storage capacity and catalytic efficiency under high-temperature conditions. Platinum remains essential for diesel engines where it catalyzes reactions at lower temperatures and enhances durability in harsher exhaust environments. The shift toward palladium in gasoline vehicles is driven by its cost-effectiveness and higher catalytic activity, improving the conversion rates of carbon monoxide, hydrocarbons, and nitrogen oxides.
Investment Potential and Market Demand
Platinum and palladium both serve as valuable investment metals, but palladium's market demand has surged due to its critical role in automotive catalytic converters, driving higher price volatility and potential returns. Platinum, with its broader industrial applications and traditional status as a luxury metal, presents a more stable investment but faces slower demand growth. Investors weigh palladium's scarcity and industrial necessity against platinum's historical value and emerging green technology applications when assessing long-term portfolio diversification.
Environmental Impact and Sustainability
Platinum extraction generates higher carbon emissions and energy consumption compared to palladium, which is often considered a more sustainable choice due to its lower environmental footprint. Palladium mining typically involves fewer toxic byproducts and reduced habitat disruption, making it preferable for eco-conscious applications. Both metals face supply chain challenges, but palladium's increasing use in catalytic converters supports its role in reducing vehicle emissions and promoting cleaner air.
Choosing Between Platinum and Palladium
Choosing between platinum and palladium depends on factors like durability, color, and price. Platinum offers superior durability and a naturally white sheen that resists tarnish, making it ideal for long-lasting jewelry. Palladium is lighter, more affordable, and shares a similar white appearance, appealing to those seeking a cost-effective yet elegant option.
Platinum Infographic
 libterm.com
libterm.com