Covalent provides a unified API that enables seamless access to blockchain data across multiple protocols, simplifying your development process. By aggregating decentralized data from various sources, Covalent ensures accurate and comprehensive insights for informed decision-making. Explore the rest of this article to discover how Covalent can enhance your blockchain applications.
Table of Comparison
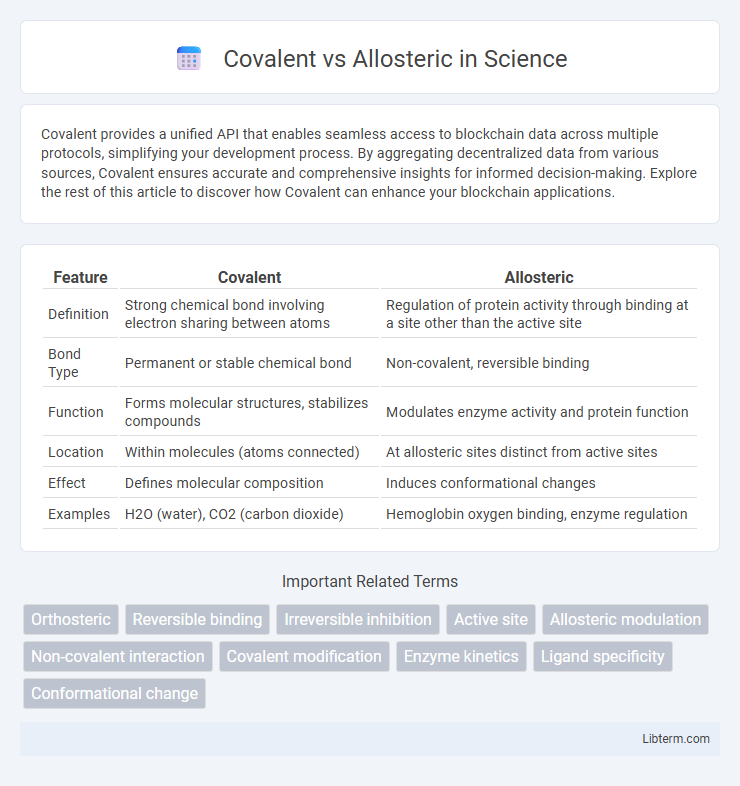
| Feature | Covalent | Allosteric |
|---|---|---|
| Definition | Strong chemical bond involving electron sharing between atoms | Regulation of protein activity through binding at a site other than the active site |
| Bond Type | Permanent or stable chemical bond | Non-covalent, reversible binding |
| Function | Forms molecular structures, stabilizes compounds | Modulates enzyme activity and protein function |
| Location | Within molecules (atoms connected) | At allosteric sites distinct from active sites |
| Effect | Defines molecular composition | Induces conformational changes |
| Examples | H2O (water), CO2 (carbon dioxide) | Hemoglobin oxygen binding, enzyme regulation |
Introduction to Covalent and Allosteric Interactions
Covalent interactions involve the sharing of electron pairs between atoms, creating strong, stable bonds that are fundamental in biochemical processes such as enzyme-substrate binding and protein function regulation. Allosteric interactions occur when molecules bind to specific sites on a protein separate from the active site, inducing conformational changes that modulate the protein's activity. Both covalent and allosteric mechanisms are essential in cellular signaling pathways, regulating enzyme activity, and maintaining metabolic control.
Defining Covalent Binding in Biochemistry
Covalent binding in biochemistry refers to the formation of stable, irreversible bonds between a ligand and its target, often through sharing electron pairs that create strong molecular connections. This type of binding contrasts with allosteric interactions, where modulators bind transiently at sites distinct from the active site, inducing conformational changes without forming permanent bonds. Covalent inhibitors are crucial in drug design for their prolonged efficacy and specificity in targeting enzymes or receptors.
Understanding Allosteric Modulation
Allosteric modulation involves the regulation of a protein's function through the binding of an effector molecule at a site distinct from the active site, causing conformational changes that alter activity. Unlike covalent modifications, which form permanent or semi-permanent bonds altering the protein structure directly, allosteric interactions are typically reversible and non-covalent, enabling dynamic control of biological pathways. This modulation plays a crucial role in fine-tuning enzyme activity, signal transduction, and receptor sensitivity, underpinning therapeutic strategies targeting allosteric sites for selective drug design.
Mechanisms of Covalent Inhibitors
Covalent inhibitors function by forming a permanent bond with a target enzyme, typically through nucleophilic attack on electrophilic groups within the active site, leading to irreversible enzyme inactivation. This mechanism often targets specific amino acid residues such as cysteine, serine, or lysine, creating a stable covalent adduct that blocks substrate access or catalytic activity. Unlike allosteric inhibitors, covalent inhibitors directly modify the enzyme's active site, resulting in prolonged inhibition due to the irreversible nature of the chemical bond.
Mechanisms of Allosteric Regulation
Allosteric regulation involves the binding of effectors to specific sites on a protein, distinct from the active site, inducing conformational changes that modulate enzymatic activity. This mechanism allows proteins to switch between active and inactive states in response to cellular signals, enabling precise control over metabolic pathways. Unlike covalent modification, which alters protein function through chemical bonds, allosteric regulation relies on non-covalent interactions that are often reversible and dynamic.
Advantages and Limitations of Covalent Drugs
Covalent drugs offer the advantage of prolonged target inhibition by forming irreversible bonds with specific enzymes or receptors, resulting in sustained therapeutic effects even at lower doses. Their high selectivity can reduce off-target interactions, minimizing side effects, but the permanent modification of target proteins raises concerns about potential toxicity and immune responses. Limitations include difficulties in dose adjustment due to irreversible binding and challenges in designing covalent inhibitors that balance efficacy with safety profiles.
Benefits and Challenges of Allosteric Modulators
Allosteric modulators offer the benefit of enhancing receptor specificity by binding to sites distinct from the orthosteric (active) site, reducing off-target effects and improving drug safety profiles. Their ability to fine-tune receptor activity allows for more precise control of signaling pathways, which is advantageous in treating complex diseases like cancer and neurological disorders. Challenges include the identification of suitable allosteric sites, variable efficacy across receptor subtypes, and potential difficulties in predicting pharmacokinetics and dynamic dosing responses.
Therapeutic Applications: Covalent vs Allosteric Agents
Covalent agents form irreversible bonds with target proteins, resulting in prolonged therapeutic effects useful for inhibiting enzymes and receptors crucial in cancer and infectious diseases treatment. Allosteric agents bind reversibly to sites distinct from the active site, modulating protein function with enhanced selectivity and reduced toxicity, ideal for neurodegenerative disorders and cardiovascular diseases. The choice between covalent and allosteric therapies depends on disease-specific targets, desired duration of action, and safety profiles in clinical applications.
Safety and Selectivity Considerations
Covalent inhibitors form irreversible bonds with target proteins, raising concerns about off-target toxicity and reduced selectivity, which can lead to adverse safety profiles. Allosteric inhibitors bind reversibly to sites distinct from the active site, offering enhanced selectivity by modulating protein function with lower risks of permanent off-target effects. Safety considerations favor allosteric modulators due to their potential for fewer side effects and better therapeutic windows in drug development.
Future Directions in Drug Design: Covalent and Allosteric Approaches
Future drug design increasingly leverages both covalent and allosteric mechanisms to enhance selectivity and efficacy. Covalent inhibitors provide prolonged target engagement through irreversible binding, offering advantages in treating diseases with traditionally undruggable proteins. Allosteric modulators enable precise regulation of protein function by binding distant sites, allowing for finely tuned therapeutic effects and reduced resistance risks, positioning both approaches as complementary strategies in next-generation pharmacotherapy.
Covalent Infographic
 libterm.com
libterm.com