Neutron emission is a type of radioactive decay where an unstable atomic nucleus releases one or more neutrons, transforming into a different isotope or element. This process plays a crucial role in nuclear reactions and is fundamental for applications in nuclear energy and medical treatments. Discover more about how neutron emission influences various scientific fields and what it means for your understanding of nuclear physics in the following article.
Table of Comparison
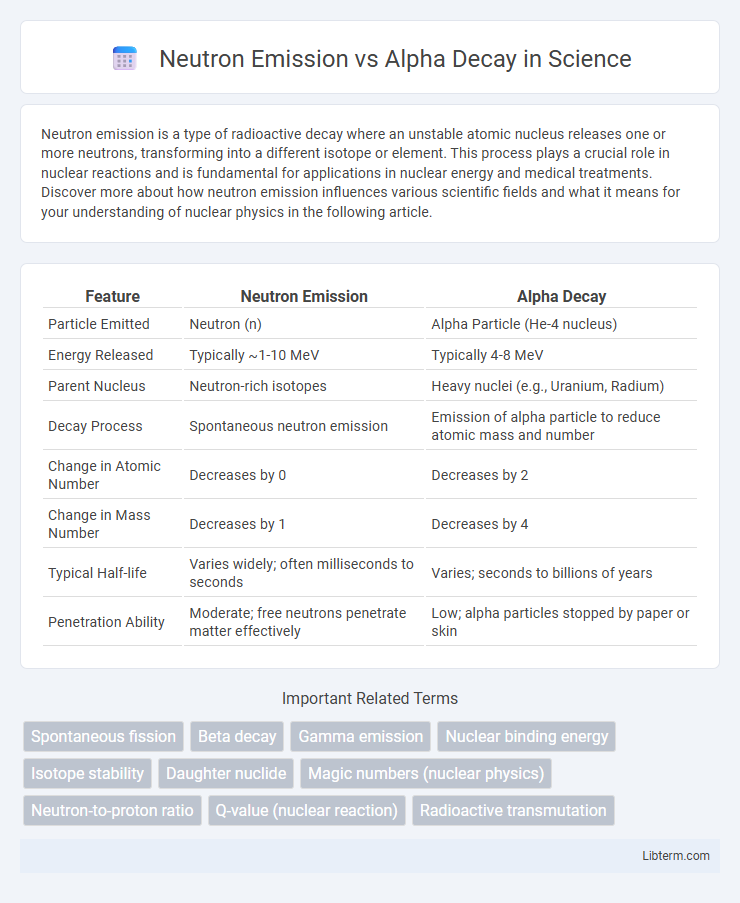
| Feature | Neutron Emission | Alpha Decay |
|---|---|---|
| Particle Emitted | Neutron (n) | Alpha Particle (He-4 nucleus) |
| Energy Released | Typically ~1-10 MeV | Typically 4-8 MeV |
| Parent Nucleus | Neutron-rich isotopes | Heavy nuclei (e.g., Uranium, Radium) |
| Decay Process | Spontaneous neutron emission | Emission of alpha particle to reduce atomic mass and number |
| Change in Atomic Number | Decreases by 0 | Decreases by 2 |
| Change in Mass Number | Decreases by 1 | Decreases by 4 |
| Typical Half-life | Varies widely; often milliseconds to seconds | Varies; seconds to billions of years |
| Penetration Ability | Moderate; free neutrons penetrate matter effectively | Low; alpha particles stopped by paper or skin |
Introduction to Nuclear Decay Processes
Nuclear decay processes involve the transformation of unstable atomic nuclei into more stable configurations by emitting particles or radiation. Neutron emission occurs when an unstable nucleus releases one or more neutrons, often in neutron-rich isotopes, leading to a change in the neutron-to-proton ratio without altering the atomic number. Alpha decay involves the emission of an alpha particle, consisting of two protons and two neutrons, which decreases both the atomic number by two and the mass number by four, commonly observed in heavy elements like uranium and radium.
Understanding Neutron Emission
Neutron emission occurs when an unstable nucleus releases one or more neutrons to achieve a more stable state, typically observed in neutron-rich isotopes beyond the neutron drip line. Unlike alpha decay, which emits a helium-4 nucleus, neutron emission results in the loss of single neutrons without altering the atomic number, thereby changing the isotope but not the element. This process plays a critical role in nuclear reactions, including those in reactors and astrophysical nucleosynthesis, due to its impact on neutron economy and elemental transmutation.
Explaining Alpha Decay
Alpha decay is a type of radioactive decay where an unstable atomic nucleus emits an alpha particle, consisting of two protons and two neutrons, effectively reducing its atomic number by two and mass number by four. This process primarily occurs in heavy elements such as uranium, thorium, and radium, leading to the formation of a new element with a lower atomic number. Alpha particles have low penetration power but can cause significant ionization, making alpha decay a key mechanism in nuclear physics and radiometric dating.
Key Differences Between Neutron Emission and Alpha Decay
Neutron emission involves the release of a neutron from an unstable nucleus, resulting in a decrease in atomic mass without changing the atomic number, while alpha decay emits an alpha particle composed of two protons and two neutrons, reducing both atomic mass and atomic number. Neutron emission typically occurs in neutron-rich isotopes near the neutron drip line, whereas alpha decay is common in heavy elements like uranium and thorium. The energy released during neutron emission is generally higher, and it affects nuclear stability differently compared to the characteristic helium nucleus emitted in alpha decay.
Energy Changes in Neutron Emission vs Alpha Decay
Neutron emission involves the release of a neutron from an unstable nucleus, often occurring in neutron-rich isotopes and resulting in smaller energy changes compared to alpha decay. Alpha decay releases an alpha particle (two protons and two neutrons), typically carrying higher kinetic energy due to the larger mass and charge of the alpha particle, with energy values generally in the range of 4 to 9 MeV. The energy difference in neutron emission versus alpha decay reflects the nuclear binding energy variations, influencing decay pathways and the stability of the resulting daughter nuclei.
Effects on Atomic Mass and Atomic Number
Neutron emission decreases the atomic mass of an atom by one unit without changing its atomic number, as a neutron is ejected from the nucleus without altering the number of protons. In contrast, alpha decay reduces the atomic mass by four units and the atomic number by two, since an alpha particle consisting of two protons and two neutrons is emitted. These differences significantly impact the resulting isotopes and elements produced during radioactive decay processes.
Stability and Occurrence in Nature
Neutron emission occurs primarily in highly neutron-rich, unstable nuclei often formed during nuclear reactions or in artificial environments, contributing to the rapid decrease in neutron excess and driving nuclei toward stability. Alpha decay, prevalent among heavy elements like uranium and thorium in nature, stabilizes the nucleus by emitting an alpha particle composed of two protons and two neutrons, reducing atomic mass and proton number simultaneously. Stability in neutron emission is less common in natural isotopes due to the immediate instability of neutron-rich nuclei, while alpha decay is a dominant natural decay mode maintaining nuclear stability of heavy elements over geologic time scales.
Technological and Scientific Applications
Neutron emission plays a critical role in nuclear reactors and neutron radiography, enabling precise material analysis and isotope production, while alpha decay is extensively used in smoke detectors and radioisotope thermoelectric generators for space missions. The high-energy neutrons emitted during neutron emission facilitate advanced imaging techniques and nuclear transmutation processes, contributing to scientific research and medical isotope generation. Alpha decay's relatively lower penetration power makes it ideal for targeted cancer therapies and radiometric dating, supporting various technological and scientific advancements.
Detection and Measurement Techniques
Neutron emission detection primarily relies on instruments such as helium-3 proportional counters, BF3 detectors, and scintillation detectors that convert neutron interactions into measurable electrical signals. Alpha decay measurement involves silicon semiconductor detectors and surface barrier detectors, which effectively identify alpha particles through their energy deposition profiles. Both processes demand careful calibration and shielding to minimize background interference and enhance measurement accuracy.
Implications for Nuclear Science and Safety
Neutron emission and alpha decay represent distinct nuclear processes with critical implications for nuclear science and safety; neutron emission releases free neutrons that can induce secondary fission in surrounding materials, significantly impacting reactor control and radiation shielding design. Alpha decay emits helium nuclei, contributing to material embrittlement and internal contamination risks but posing limited external radiation hazard due to low penetration depth. Understanding the differential effects of neutron emission and alpha decay enables precise risk assessment, optimized containment strategies, and enhanced safety protocols in nuclear reactors and radioactive waste management.
Neutron Emission Infographic
 libterm.com
libterm.com