Dilute refers to the process of reducing the concentration of a substance in a solution, often by adding more solvent like water. This technique is commonly used in chemistry, cooking, and medicine to achieve desired strength or potency. Explore the rest of the article to understand how dilution impacts various applications and how you can effectively use it.
Table of Comparison
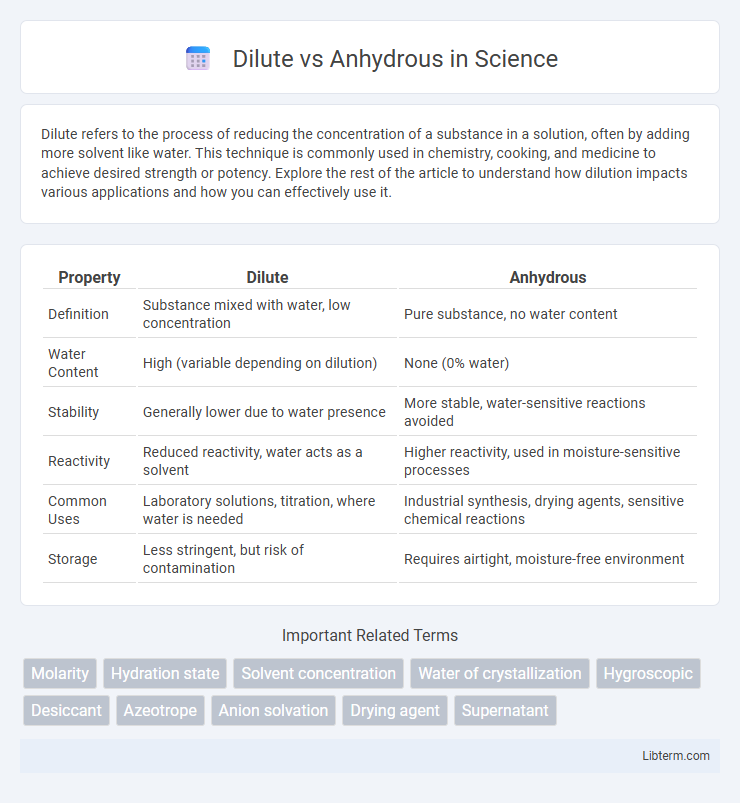
| Property | Dilute | Anhydrous |
|---|---|---|
| Definition | Substance mixed with water, low concentration | Pure substance, no water content |
| Water Content | High (variable depending on dilution) | None (0% water) |
| Stability | Generally lower due to water presence | More stable, water-sensitive reactions avoided |
| Reactivity | Reduced reactivity, water acts as a solvent | Higher reactivity, used in moisture-sensitive processes |
| Common Uses | Laboratory solutions, titration, where water is needed | Industrial synthesis, drying agents, sensitive chemical reactions |
| Storage | Less stringent, but risk of contamination | Requires airtight, moisture-free environment |
Introduction to Dilute and Anhydrous Substances
Dilute substances contain a lower concentration of solute dissolved in a solvent, resulting in a less intense chemical solution commonly used in laboratory and industrial applications. Anhydrous substances, on the other hand, are compounds completely free of water molecules and are essential in chemical reactions where moisture can cause unwanted side effects or interfere with results. Understanding the differences between dilute and anhydrous forms is crucial for precise control of chemical properties and reaction outcomes.
Defining Dilute: Meaning and Examples
Dilute solutions contain a relatively small amount of solute dispersed in a larger volume of solvent, reducing the concentration compared to more concentrated or anhydrous forms. For example, dilute hydrochloric acid might have a concentration of 0.1 M, making it less reactive and safer for certain applications than its concentrated counterpart. Understanding the meaning of dilute helps distinguish it from anhydrous substances, which contain no water and often exhibit different chemical properties.
What Does Anhydrous Mean? Key Characteristics
Anhydrous refers to a substance that contains no water molecules within its crystalline structure or composition, often found in chemical compounds like anhydrous calcium sulfate or anhydrous ammonia. Key characteristics of anhydrous materials include their stability and higher purity compared to their hydrated counterparts, as the absence of water reduces the risk of chemical reactions involving moisture. Anhydrous substances typically require specific storage conditions to prevent absorption of moisture from the environment, which can alter their physical and chemical properties.
Chemical Properties: Dilute vs Anhydrous
Dilute solutions contain a lower concentration of solute molecules dispersed in a solvent, resulting in weaker intermolecular forces and reduced reactivity compared to anhydrous forms. Anhydrous substances lack water molecules, exhibiting stronger ionic or covalent bonding and higher thermal stability, which significantly alters their chemical behavior. The presence or absence of water in these substances directly influences properties such as solubility, reactivity, and stability.
Common Applications in Industry and Laboratories
Dilute solutions are widely used in industries such as pharmaceuticals for precise drug formulation and in laboratories for titration and chemical analysis where controlled concentration is essential. Anhydrous compounds find critical applications in chemical manufacturing, particularly in moisture-sensitive reactions like organic synthesis and electronic materials production, where water can cause product degradation. Both forms are integral to industrial and laboratory processes, with dilute solutions facilitating safe handling and measurement, and anhydrous substances ensuring purity and reactivity.
Importance of Water Content in Chemical Reactions
Water content significantly influences chemical reaction rates and mechanisms, as dilute solutions often alter reactant solubility and ionization compared to anhydrous conditions. Anhydrous reagents provide precise stoichiometry and prevent unwanted hydrolysis or side reactions, crucial in sensitive syntheses like organometallic chemistry. Controlling moisture ensures reproducibility and selectivity, impacting yield and product purity in both academic and industrial applications.
Storage and Safety Considerations
Dilute solutions require storage in corrosion-resistant containers and should be kept away from incompatible materials due to their lower concentration but increased volume, posing spill risks. Anhydrous substances demand airtight, moisture-free storage conditions to prevent hydrolysis and maintain stability, often in cool, dry environments. Both forms necessitate proper labeling, use of personal protective equipment (PPE), and adherence to safety data sheets (SDS) to minimize hazards during handling and storage.
Impact on Solubility and Reactivity
Dilute solutions contain a lower concentration of solute, which generally leads to increased solubility due to a greater solvent-to-solute ratio, facilitating easier dissolution. In contrast, anhydrous substances lack water, often resulting in decreased solubility in aqueous environments but can exhibit higher reactivity in non-aqueous or controlled conditions due to the absence of hydration effects. The presence or absence of water significantly influences reaction mechanisms, with anhydrous compounds often undergoing more rapid or different chemical transformations compared to their dilute, hydrated counterparts.
Dilute and Anhydrous in Everyday Life
Dilute solutions, commonly found in household cleaning products and beverages, contain a small amount of solute dispersed in a large volume of solvent, making them safer and easier to handle in daily use. Anhydrous substances, such as anhydrous ethanol or anhydrous calcium chloride, are vital in industrial applications and pharmaceutical formulations due to their lack of water content, which prevents unwanted reactions and degradation. Understanding the difference between dilute and anhydrous helps consumers and professionals choose the right form for cooking, cleaning, or chemical processes.
Conclusion: Choosing Between Dilute and Anhydrous
Selecting between dilute and anhydrous forms depends primarily on the specific application requirements and chemical properties. Dilute solutions are favored for safer handling and controlled reactions, while anhydrous compounds are essential when moisture-free conditions are critical to prevent unwanted side reactions. Evaluating factors such as reactivity, storage conditions, and desired precision determines the optimal choice for laboratory or industrial use.
Dilute Infographic
 libterm.com
libterm.com