A reducer is a mechanical device used to decrease the speed and increase the torque of a motor or engine, optimizing power transmission in various machinery. Its design ensures efficient energy transfer, enhancing the performance and longevity of equipment in industries like manufacturing and automotive. Explore the detailed functions and types of reducers to understand how they can improve your mechanical systems.
Table of Comparison
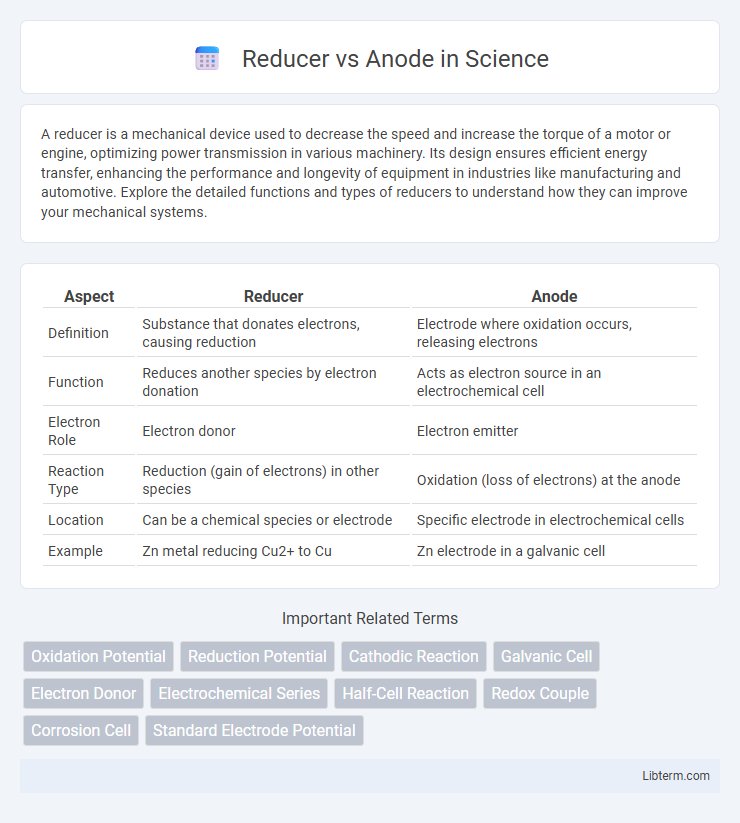
| Aspect | Reducer | Anode |
|---|---|---|
| Definition | Substance that donates electrons, causing reduction | Electrode where oxidation occurs, releasing electrons |
| Function | Reduces another species by electron donation | Acts as electron source in an electrochemical cell |
| Electron Role | Electron donor | Electron emitter |
| Reaction Type | Reduction (gain of electrons) in other species | Oxidation (loss of electrons) at the anode |
| Location | Can be a chemical species or electrode | Specific electrode in electrochemical cells |
| Example | Zn metal reducing Cu2+ to Cu | Zn electrode in a galvanic cell |
Introduction to Reducers and Anodes
Reducers are chemical agents that donate electrons in redox reactions, playing a crucial role in processes like metal extraction and corrosion prevention. Anodes serve as the electrode where oxidation occurs, releasing electrons into an external circuit or surrounding environment. Understanding the functions of reducers and anodes is essential in electrochemistry, especially for applications involving batteries, electrolytic cells, and corrosion control systems.
Core Functions: Reducer vs Anode
The core function of a reducer in chemical reactions or electrochemical cells is to donate electrons, thereby reducing another substance by increasing its oxidation state. In contrast, the anode serves as the electrode where oxidation occurs, releasing electrons into the external circuit. While the reducer is defined by its chemical capacity to reduce, the anode is a physical component that facilitates oxidation and electron flow within electrochemical systems.
Key Differences in Application
Reducers serve as electron donors in redox reactions, facilitating reduction processes primarily in chemical synthesis and energy storage systems like batteries. Anodes function as the electrode where oxidation occurs, essential in electrochemical cells for electron flow direction and corrosion prevention. The key difference lies in reducers being chemical species involved in electron transfer, whereas anodes are physical components in devices controlling the electrochemical reactions.
Material Composition and Properties
Reducers typically consist of carbon steel or stainless steel alloys designed for strength and corrosion resistance in piping systems. Anodes are usually made from sacrificial metals such as zinc, magnesium, or aluminum alloys, chosen for their electrochemical properties that promote corrosion protection. Material composition directly influences durability, with reducers emphasizing mechanical stability and anodes focusing on reactive properties for cathodic protection.
Common Industrial Uses
Reducers and anodes serve distinct purposes in industrial applications, with reducers commonly used in piping systems to connect pipes of different diameters, ensuring smooth fluid flow and pressure management in oil and gas, chemical processing, and water treatment industries. Anodes are primarily employed in cathodic protection systems to prevent corrosion in metal structures such as pipelines, tanks, and marine vessels, extending their lifespan in industries like offshore drilling, power generation, and wastewater management. Both components are critical in maintaining equipment integrity and operational efficiency in harsh industrial environments.
Advantages of Using Reducers
Reducers enhance system efficiency by providing precise control over rotational speed and torque, which improves mechanical performance in various industrial applications. They reduce energy consumption and wear on equipment, extending the lifespan of machinery and lowering maintenance costs. Using reducers also allows for compact design integration, saving space while optimizing power transmission in manufacturing and automation processes.
Benefits of Deploying Anodes
Deploying anodes offers significant benefits such as enhanced corrosion protection by serving as a controlled sacrificial metal that corrodes instead of the protected structure, thereby extending the lifespan of pipelines, tanks, and marine vessels. Anodes also provide cost-effective maintenance by reducing the frequency of repairs and inspections needed due to corrosion damage. Their targeted protection capabilities enable precise control over corrosion processes, improving overall asset integrity and safety.
Selection Criteria for Reducers and Anodes
Selection criteria for reducers and anodes depend on their electrochemical properties and application requirements. Reducers must have a suitable reduction potential, stability in the operating environment, and compatibility with other materials to ensure efficient electron transfer. Anodes are chosen based on their corrosion resistance, sacrificial activity, and ability to provide consistent protective current in cathodic protection systems.
Maintenance and Lifespan Comparison
Reducers generally require less maintenance due to their simple mechanical design, whereas anodes need regular inspection and replacement to prevent corrosion effectively. The lifespan of reducers tends to be longer if properly lubricated and not exposed to extreme conditions, while anodes have a limited lifespan as they sacrifice material to protect metal structures. Regular maintenance of anodes is critical to ensure optimal performance and extend the service life of the protected equipment.
Conclusion: Choosing Between Reducer and Anode
Selecting between a reducer and an anode depends primarily on the intended application and electrochemical environment. Reducers serve to gain electrons in redox reactions, crucial for chemical synthesis and energy storage systems, while anodes are key in corrosion protection and electrochemical cells, acting as the site of oxidation. Optimal choice hinges on specific functional requirements, such as electron flow direction and material compatibility within the system.
Reducer Infographic
 libterm.com
libterm.com