Acid-base titration is a quantitative analytical method used to determine the concentration of an unknown acid or base by neutralizing it with a base or acid of known concentration. During the titration, a pH indicator or pH meter signals the endpoint, allowing precise calculation of your solution's molarity. Explore the full article to master the steps and applications of acid-base titrations.
Table of Comparison
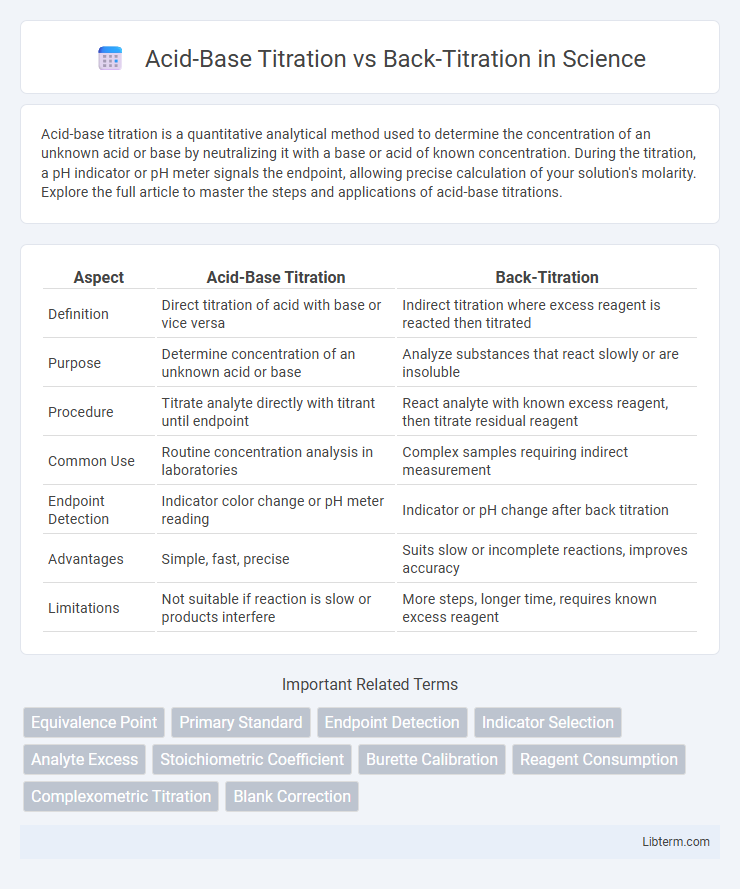
| Aspect | Acid-Base Titration | Back-Titration |
|---|---|---|
| Definition | Direct titration of acid with base or vice versa | Indirect titration where excess reagent is reacted then titrated |
| Purpose | Determine concentration of an unknown acid or base | Analyze substances that react slowly or are insoluble |
| Procedure | Titrate analyte directly with titrant until endpoint | React analyte with known excess reagent, then titrate residual reagent |
| Common Use | Routine concentration analysis in laboratories | Complex samples requiring indirect measurement |
| Endpoint Detection | Indicator color change or pH meter reading | Indicator or pH change after back titration |
| Advantages | Simple, fast, precise | Suits slow or incomplete reactions, improves accuracy |
| Limitations | Not suitable if reaction is slow or products interfere | More steps, longer time, requires known excess reagent |
Introduction to Acid-Base Titration
Acid-base titration is a quantitative analytical technique used to determine the concentration of an unknown acid or base by reacting it with a standard solution of base or acid of known concentration. The process involves the gradual addition of the titrant to the analyte until the reaction reaches its equivalence point, which is often indicated by a pH indicator or a pH meter. This method provides precise measurements essential for applications in chemistry, pharmaceuticals, and environmental analysis.
Fundamentals of Back-Titration
Back-titration is a quantitative analytical technique used when the analyte is insoluble, volatile, or when the reaction between the analyte and the titrant is slow or incomplete. In back-titration, a known excess of standard reagent is added to the analyte, and the unreacted excess is titrated with a second titrant to determine the amount consumed in the initial reaction. This method provides accurate results in cases where direct titration is challenging, allowing for precise quantification of substances through indirect measurement.
Key Differences Between Acid-Base and Back-Titration
Acid-base titration directly measures the concentration of an acid or base by reacting it with a standard solution until the equivalence point is reached. In contrast, back-titration involves adding an excess reagent to the analyte and then titrating the remaining reagent to determine the amount consumed. The key differences include direct endpoint determination in acid-base titration versus indirect measurement in back-titration, suitability of back-titration for insoluble or slow-reacting substances, and the increased complexity of calculations in back-titration compared to straightforward acid-base titration.
When to Use Acid-Base Titration
Acid-base titration is ideal for analyzing samples with clear stoichiometric neutralization reactions, such as strong acid-strong base or weak acid-strong base combinations, where the endpoint can be accurately detected using indicators or pH meters. This method is preferred when the analyte is directly reactive with the titrant and the reaction reaches completion in a reasonable time, allowing precise determination of concentration. It is commonly used in quality control of pharmaceuticals, water analysis, and food industry where simplicity and speed are prioritized.
Applications of Back-Titration in Analytical Chemistry
Back-titration is extensively employed in analytical chemistry to determine the concentration of substances that react slowly or are insoluble in water, such as certain metal oxides and pharmaceutical compounds. It is particularly useful for analyzing samples that are difficult to dissolve or when direct titration is impractical, including soil samples, antacids, and complex biological materials. This method enhances accuracy and efficiency in quantifying analytes by reacting the sample with an excess reagent and subsequently titrating the remaining unreacted reagent.
Advantages and Limitations of Acid-Base Titration
Acid-base titration offers precise determination of unknown concentrations through direct neutralization, with clear end-point detection using pH indicators or pH meters. Limitations include difficulty in analyzing weak acids or bases due to less distinct equivalence points and challenges in titrating substances that react slowly or form intermediates. While cost-effective and straightforward, acid-base titration may require stringent control of experimental conditions to ensure accuracy, especially in complex matrices.
Benefits and Drawbacks of Back-Titration
Back-titration offers enhanced accuracy when direct titration is impractical, especially for substances that react slowly or form insoluble precipitates. This method allows analysis of samples with low solubility or weak acidic or basic properties, making it versatile for complex mixtures. However, back-titration involves more procedural steps and can introduce cumulative errors, requiring careful endpoint determination and increased laboratory time compared to standard acid-base titration.
Step-by-Step Procedures for Both Titration Methods
In acid-base titration, a measured volume of acid or base is gradually added to a known volume of the opposite solution until the equivalence point is reached, indicated by a pH indicator or pH meter. Back-titration involves adding an excess amount of standard reagent to the analyte, then titrating the remaining excess with another standard solution to determine the analyte concentration indirectly. Both methods require careful preparation of standard solutions, accurate volume measurements using burettes, and precise endpoint detection to ensure reliable and reproducible results.
Accuracy, Precision, and Error Sources
Acid-base titration offers high accuracy and precision when the endpoint is clear and the standard solution concentration is well-known, minimizing systematic errors. Back-titration enhances accuracy in cases where the analyte is insoluble or reacts slowly, reducing errors linked to incomplete reactions or endpoint detection difficulties. Error sources in both methods include improper indicator choice, titrant standardization inaccuracies, and human reading errors, with back-titration adding complexity that may introduce additional procedural uncertainties.
Choosing the Right Method: Acid-Base Titration vs Back-Titration
Selecting between acid-base titration and back-titration depends on the nature of the analyte and reaction speed; acid-base titration suits straightforward, fast reactions with clear endpoints. Back-titration is preferred for weak acids or bases, insoluble substances, or when the endpoint is difficult to detect directly, enhancing accuracy. Understanding sample composition and reaction kinetics ensures optimal method choice for precise quantitative analysis.
Acid-Base Titration Infographic
 libterm.com
libterm.com