Acyclic structures play a crucial role in various scientific and computational fields, particularly in graph theory and chemistry where they represent non-repetitive, tree-like configurations. Understanding acyclic properties helps in optimizing network designs and analyzing molecular compounds accurately. Explore the full article to see how acyclic principles impact your area of interest and practical applications.
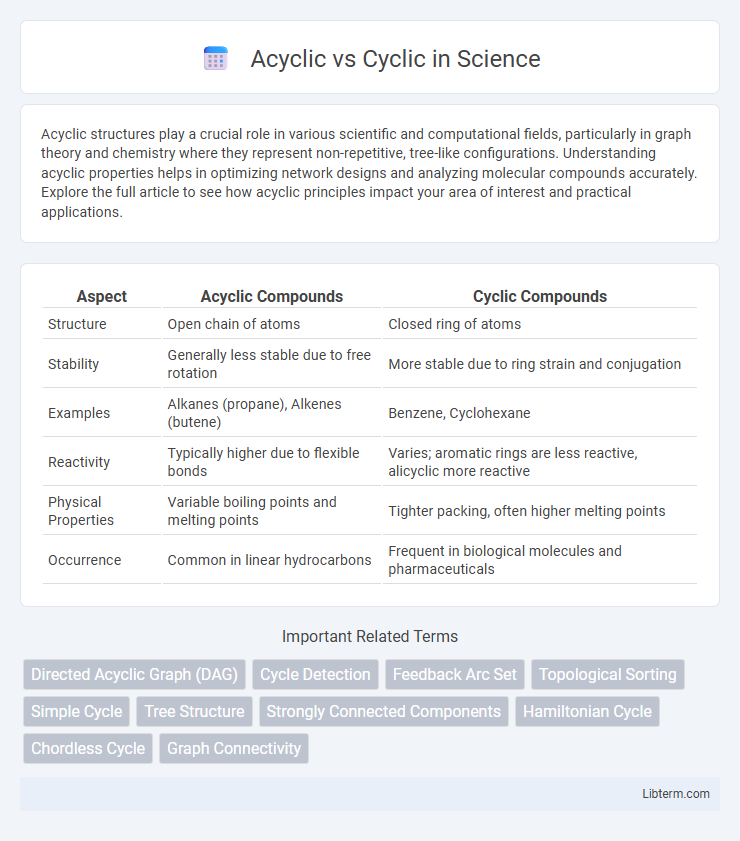
Table of Comparison
| Aspect | Acyclic Compounds | Cyclic Compounds |
|---|---|---|
| Structure | Open chain of atoms | Closed ring of atoms |
| Stability | Generally less stable due to free rotation | More stable due to ring strain and conjugation |
| Examples | Alkanes (propane), Alkenes (butene) | Benzene, Cyclohexane |
| Reactivity | Typically higher due to flexible bonds | Varies; aromatic rings are less reactive, alicyclic more reactive |
| Physical Properties | Variable boiling points and melting points | Tighter packing, often higher melting points |
| Occurrence | Common in linear hydrocarbons | Frequent in biological molecules and pharmaceuticals |
Introduction to Acyclic and Cyclic Structures
Acyclic structures are characterized by the absence of closed loops, making them essential in applications like tree data models and hierarchical networks where clear, non-repetitive paths are crucial. Cyclic structures contain one or more loops, enabling complex relationships and feedback mechanisms commonly found in graph theory, molecular chemistry, and circuit design. Understanding the fundamental differences between acyclic and cyclic structures aids in selecting appropriate models for computational processes, data organization, and network topology.
Definitions and Core Concepts
Acyclic structures are graphs or frameworks that contain no cycles, meaning there are no closed loops within the connections between nodes. Cyclic structures, in contrast, include at least one cycle where a path starts and ends at the same node, forming a closed loop. Understanding the presence or absence of cycles is fundamental in fields like graph theory, computer science, and chemistry for analyzing network flow, data structures, and molecular stability.
Key Differences Between Acyclic and Cyclic
Acyclic structures contain no cycles, meaning their paths do not loop back to the starting point, while cyclic structures include at least one cycle, forming closed loops. In graph theory, acyclic graphs such as trees have a hierarchical organization and guarantee no repetition along any path, whereas cyclic graphs allow for repeated traversal through nodes due to the presence of cycles. This fundamental difference impacts algorithms for traversal, detection, and processing, with acyclic graphs supporting simpler execution for tasks like topological sorting, which is impossible in cyclic graphs.
Examples of Acyclic Structures
Acyclic structures are exemplified by trees in computer science, where nodes are connected without forming any loops, such as binary trees used in search algorithms. Another example is Directed Acyclic Graphs (DAGs), commonly applied in project scheduling with tasks ordered without circular dependencies. Chemical compounds like alkanes also represent acyclic structures, consisting of carbon atoms arranged in open chains without rings.
Examples of Cyclic Structures
Cyclic structures are molecules that contain one or more rings formed by atoms connected in a closed loop, such as benzene (C6H6), cyclohexane (C6H12), and naphthalene (C10H8). These compounds exhibit unique chemical properties due to the ring strain and resonance effects present in cyclic arrangements, influencing their reactivity and stability. Examples of cyclic structures extend to biological molecules like glucose, which contains a six-membered ring, and DNA, featuring cyclic sugar-phosphate backbones essential for genetic information storage.
Chemical Properties: Acyclic vs Cyclic
Acyclic compounds consist of open-chain structures with no rings, resulting in greater conformational flexibility and typically higher reactivity due to accessible functional groups. Cyclic compounds form ring structures that introduce strain and rigidity, influencing their chemical properties such as stability, reactivity, and melting points. The ring strain in small cyclic molecules often increases reactivity, while larger rings exhibit properties closer to acyclic analogs, impacting their chemical behavior and synthesis pathways.
Applications in Organic Chemistry
Acyclic compounds, characterized by open-chain structures, are widely used in pharmaceutical synthesis due to their flexibility and ease of functionalization, enabling targeted drug development. Cyclic compounds, including aromatic and non-aromatic rings, play crucial roles in medicinal chemistry and materials science, providing structural rigidity and unique electronic properties important for enzyme inhibition and polymer design. Understanding the differences in reactivity and stability between acyclic and cyclic molecules guides chemists in optimizing reaction pathways and designing novel organic compounds.
Advantages and Disadvantages
Acyclic structures, such as trees and directed acyclic graphs (DAGs), provide advantages like simplified data processing, easier detection of dependencies, and prevention of infinite loops, making them ideal for scheduling and hierarchy representation. Cyclic structures, including graphs with cycles, allow modeling of complex relationships and feedback systems, supporting processes like network routing and dynamic system simulations, though they pose challenges in detecting cycles and risk of infinite recursion. The main disadvantage of acyclic models is their inability to represent repetitive or circular relationships, while cyclic models require sophisticated algorithms to manage loops and ensure termination.
Common Mistakes in Identification
Confusing acyclic and cyclic structures often occurs due to overlooking subtle ring closures in molecules or graphs, leading to incorrect classification. A common mistake is misidentifying complex branched acyclic systems as cyclic when hidden cycles exist, or vice versa when apparent loops are merely overlapping chains. Precise analysis tools such as depth-first search in graph theory or careful ring detection algorithms in chemistry help reduce these errors significantly.
Conclusion and Comparative Summary
Acyclic structures lack closed loops, providing simpler, more predictable pathways essential in data processing and chemical reactions, while cyclic structures contain one or more loops, offering stability and unique properties such as resonance in molecules or feedback in systems. The absence of cycles in acyclic entities ensures efficient traversal and easier computational analysis, contrasting with the complexity and potential redundancies introduced by cyclic formations. Choosing between acyclic and cyclic depends on application requirements, favoring acyclic for streamlined processes and cyclic for enhanced stability and dynamic behavior.
Acyclic Infographic
 libterm.com
libterm.com