Complexometric titration accurately determines metal ion concentrations by forming stable complexes with a chelating agent, often EDTA, under controlled pH conditions. It is widely used in water analysis, pharmaceuticals, and food industries due to its precision and minimal interference from other ions. Explore the rest of this article to understand the principles, procedures, and applications of complexometric titration.
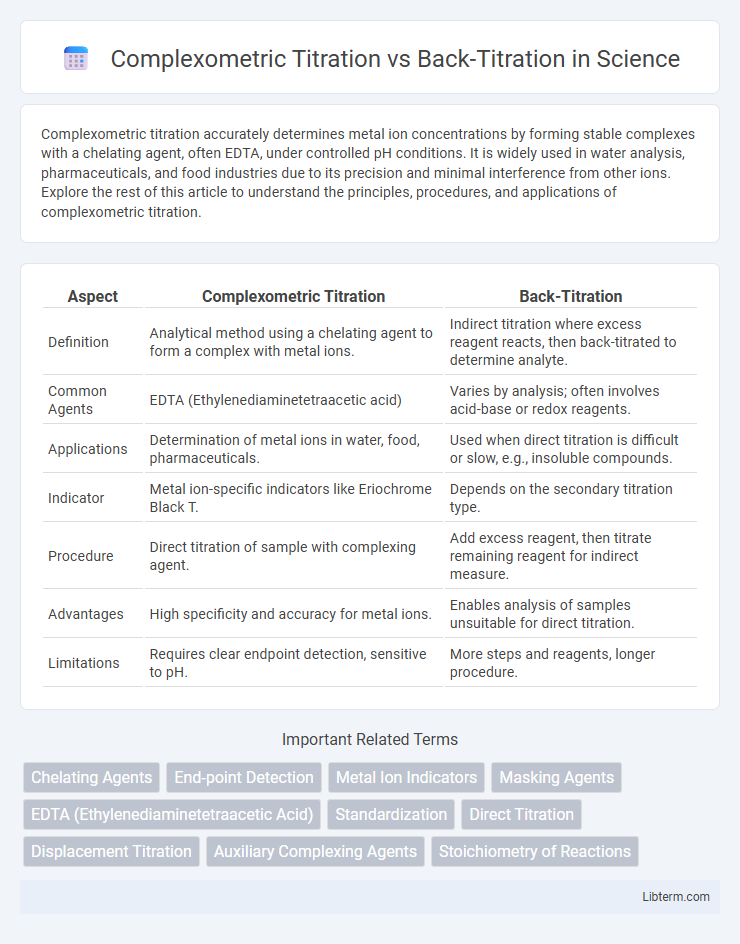
Table of Comparison
| Aspect | Complexometric Titration | Back-Titration |
|---|---|---|
| Definition | Analytical method using a chelating agent to form a complex with metal ions. | Indirect titration where excess reagent reacts, then back-titrated to determine analyte. |
| Common Agents | EDTA (Ethylenediaminetetraacetic acid) | Varies by analysis; often involves acid-base or redox reagents. |
| Applications | Determination of metal ions in water, food, pharmaceuticals. | Used when direct titration is difficult or slow, e.g., insoluble compounds. |
| Indicator | Metal ion-specific indicators like Eriochrome Black T. | Depends on the secondary titration type. |
| Procedure | Direct titration of sample with complexing agent. | Add excess reagent, then titrate remaining reagent for indirect measure. |
| Advantages | High specificity and accuracy for metal ions. | Enables analysis of samples unsuitable for direct titration. |
| Limitations | Requires clear endpoint detection, sensitive to pH. | More steps and reagents, longer procedure. |
Introduction to Complexometric Titration
Complexometric titration is a quantitative analytical technique used to determine metal ion concentrations through the formation of stable complexes with specific chelating agents like EDTA. This method relies on the precise stoichiometric reaction between the metal ions and the complexing agent, which can be monitored using suitable indicators or potentiometric sensors. It is widely applied in water hardness testing, pharmaceutical analysis, and metallurgical processes for its accuracy and specificity.
Basics of Back-Titration Explained
Back-titration is a quantitative analytical technique where an excess amount of standard reagent is added to the analyte, and the leftover reagent is titrated with another standard solution. Unlike complexometric titration, which directly measures metal ions through complex formation, back-titration indirectly determines the analyte concentration by calculating the unreacted reagent. This method is particularly useful for samples that react slowly or are insoluble, providing more accurate and reliable results in such cases.
Key Differences Between Complexometric and Back-Titration
Complexometric titration involves the direct formation of a stable complex between a metal ion and a chelating agent such as EDTA, allowing precise determination of metal ion concentration through stoichiometric reactions. Back-titration, on the other hand, is an indirect analytical technique where an excess of a standard reagent is added to react with the analyte, and the unreacted excess is titrated with a second reagent to calculate the analyte concentration. Key differences lie in the methodology: complexometric titration measures the analyte directly using complex formation, while back-titration quantifies analyte concentration indirectly through residual reagent measurement after a reaction.
Reagents and Indicators Used
Complexometric titration primarily uses EDTA as the reagent to form stable complexes with metal ions, while indicators such as Eriochrome Black T or Calcein are employed to signal the end point by changing color upon metal ion binding. Back-titration involves an initial excess of a standard reagent like EDTA or a strong acid/base reacting with the analyte, followed by titration of the unreacted reagent with a secondary titrant; indicators like Murexide or Starch are chosen based on the specific reaction and analyte. The selection of reagents and indicators in both methods depends on the metal ion's chemical properties and the desired accuracy of the titration process.
Applications in Analytical Chemistry
Complexometric titration is widely used for determining metal ions in water hardness analysis, alloy composition, and pharmaceutical formulations due to its precision in forming stable metal-ligand complexes. Back-titration is preferred when the analyte reacts slowly or is insoluble, making it ideal for analyzing carbonate content in cement, antacid potency, and determining fatty acid concentrations in oils. Both techniques enhance accuracy in quantitative chemical analysis by adapting to different sample properties and reaction kinetics.
Procedure and Methodology Comparison
Complexometric titration involves directly adding a chelating agent like EDTA to form a stable complex with metal ions in solution, monitored by a suitable indicator until the endpoint is reached. In contrast, back-titration first reacts the analyte with an excess of a standard reagent, and the remaining reagent is then titrated with another standard solution to determine the analyte concentration indirectly. The procedure of complexometric titration is straightforward and often faster, while back-titration suits analyses where direct titration is impractical due to slow reaction rates or insoluble products.
Advantages and Disadvantages of Each Method
Complexometric titration offers precise determination of metal ion concentrations using chelating agents like EDTA, allowing rapid and straightforward analysis with minimal interferences. However, its accuracy can be compromised by metal ion complex stability and pH sensitivity, requiring careful control of experimental conditions. Back-titration serves well for analytes that react slowly or form weak complexes by reacting the excess reagent and titrating it, but this method involves additional steps and potential cumulative errors, making it less direct and more time-consuming.
Accuracy and Precision Considerations
Complexometric titration offers high accuracy in determining metal ion concentrations by forming stable, specific complexes, minimizing interference from other ions. Back-titration enhances precision when direct titration is challenging due to slow reaction rates or insoluble products, allowing more controlled endpoint detection. Both methods require careful reagent standardization and endpoint identification to achieve optimal analytical reliability.
Common Errors and Troubleshooting
Common errors in complexometric titration include inaccurate endpoint detection due to improper indicator selection or incomplete complex formation, often leading to erroneous metal ion concentration results. In back-titration, mistakes such as insufficient reaction time, incorrect reagent volume, or neglecting to account for side reactions can skew final calculations. Troubleshooting involves careful calibration of indicators, ensuring complete reaction by allowing adequate time, and thorough standardization of all reagents to improve titration accuracy.
Conclusion: Choosing the Right Titration Method
Complexometric titration is ideal for directly determining metal ion concentrations using specific chelating agents like EDTA, offering high precision for water hardness and metal analysis. Back-titration is preferred when the analyte is insoluble, slow to react, or when the endpoint of the main reaction is difficult to observe, improving accuracy by reacting excess reagent with a secondary titrant. Selecting the right method depends on the sample's chemical properties, reaction kinetics, and the ability to clearly detect the equivalence point.
Complexometric Titration Infographic
 libterm.com
libterm.com