Hydrophilic substances have an affinity for water, meaning they readily absorb or dissolve in it due to their polar nature. These molecules play a crucial role in various biological, chemical, and industrial processes, influencing how materials interact with moisture. Explore the rest of the article to discover how hydrophilic properties impact your daily life and technological applications.
Table of Comparison
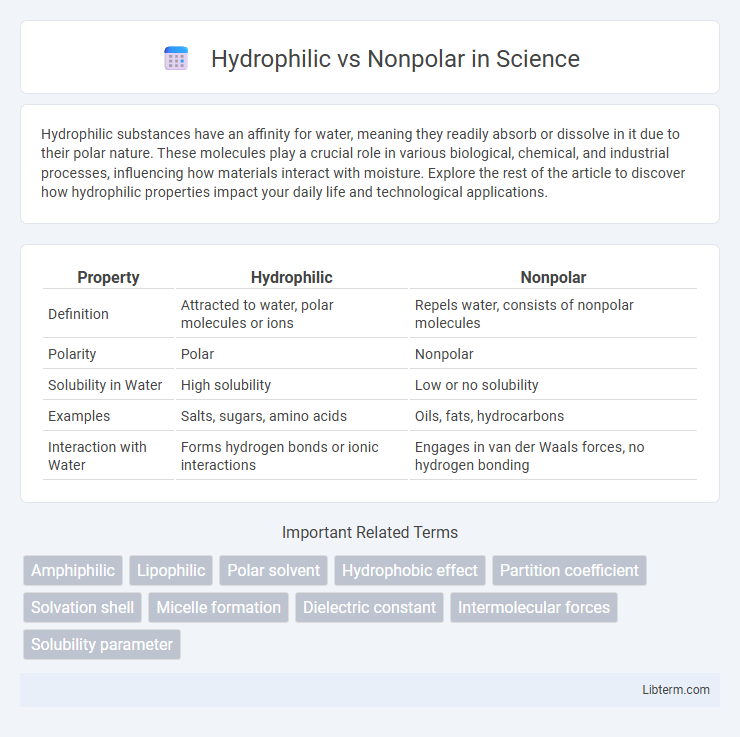
| Property | Hydrophilic | Nonpolar |
|---|---|---|
| Definition | Attracted to water, polar molecules or ions | Repels water, consists of nonpolar molecules |
| Polarity | Polar | Nonpolar |
| Solubility in Water | High solubility | Low or no solubility |
| Examples | Salts, sugars, amino acids | Oils, fats, hydrocarbons |
| Interaction with Water | Forms hydrogen bonds or ionic interactions | Engages in van der Waals forces, no hydrogen bonding |
Introduction to Hydrophilic and Nonpolar Concepts
Hydrophilic molecules possess polar functional groups that enable them to form hydrogen bonds with water, resulting in high solubility in aqueous environments. Nonpolar molecules lack these polar regions, causing them to be insoluble in water but soluble in nonpolar solvents due to London dispersion forces. Understanding the distinct interactions of hydrophilic and nonpolar substances is crucial in fields like biochemistry, pharmaceuticals, and environmental science.
Defining Hydrophilic Substances
Hydrophilic substances contain polar groups or charged atoms that can form hydrogen bonds with water molecules, resulting in strong attraction and solubility in aqueous solutions. Common examples include salts, alcohols, and sugars, which readily dissolve due to their affinity for water's polarity. These molecules typically feature functional groups like hydroxyl (-OH), carboxyl (-COOH), or amino (-NH2) that increase their hydrophilicity compared to nonpolar substances.
Understanding Nonpolar Compounds
Nonpolar compounds consist of molecules with evenly distributed electrical charges, resulting in an absence of significant dipole moments. These substances, primarily composed of carbon and hydrogen atoms, do not interact favorably with water due to the lack of polarity, causing them to be hydrophobic. Understanding nonpolar compounds is crucial in fields such as biochemistry and pharmacology, where their behavior influences membrane formation and drug solubility.
Molecular Structure and Polarity
Hydrophilic molecules contain polar functional groups such as hydroxyl (-OH) or amine (-NH2), enabling them to form hydrogen bonds with water, resulting in high solubility. Nonpolar molecules consist mainly of nonpolar covalent bonds, often composed of hydrocarbons, which lack significant electronegativity differences and do not interact favorably with polar water molecules. The difference in molecular polarity dictates their solubility behavior, with hydrophilic substances being water-attracting and nonpolar substances being water-repellent.
Water Solubility: Hydrophilic vs Nonpolar
Hydrophilic molecules exhibit high water solubility due to their polar nature and ability to form hydrogen bonds with water molecules. Nonpolar molecules lack this polarity, resulting in poor water solubility because they cannot effectively interact with the polar water environment. This fundamental difference in molecular structure dictates the distinct solubility behaviors of hydrophilic versus nonpolar substances in aqueous solutions.
Chemical Examples: Hydrophilic and Nonpolar Molecules
Hydrophilic molecules, such as glucose and ethanol, contain polar functional groups like hydroxyl (-OH) that form hydrogen bonds with water, enhancing their solubility. Nonpolar molecules like hexane and benzene consist mainly of carbon-hydrogen bonds, lacking polarity, which makes them insoluble in water but soluble in nonpolar solvents. Understanding these chemical examples highlights how molecular polarity influences solubility and interactions in different environments.
Biological Significance of Hydrophilic and Nonpolar Properties
Hydrophilic molecules, characterized by their affinity for water, play essential roles in biological systems such as facilitating nutrient transport and enzyme activity within aqueous environments. Nonpolar molecules, lacking charge and polarity, are critical in forming cellular membranes through hydrophobic interactions, maintaining structural integrity and selective permeability. The balance between hydrophilic and nonpolar properties drives protein folding, membrane dynamics, and molecular recognition processes vital for cellular function.
Industrial Applications and Uses
Hydrophilic materials, characterized by their affinity for water, are extensively used in industries such as pharmaceuticals, water treatment, and textiles for applications like drug delivery systems, membrane filtration, and moisture-wicking fabrics. Nonpolar substances, which repel water and exhibit strong interactions with other nonpolar compounds, are crucial in sectors like lubricants, coatings, and petrochemicals for formulating greases, waterproof paints, and fuel additives. The distinct molecular interactions of hydrophilic and nonpolar compounds dictate their selection in industrial processes to optimize performance and durability under specific environmental conditions.
Key Differences between Hydrophilic and Nonpolar
Hydrophilic molecules possess polar bonds or charged groups that enable strong interactions with water through hydrogen bonding or ion-dipole forces, making them water-soluble. Nonpolar molecules lack significant charge differences and have symmetrical electron distribution, resulting in weak interactions with water and poor solubility. These fundamental differences influence their behavior in biological systems, such as membrane formation and protein folding, where hydrophilic regions interact with aqueous environments, and nonpolar regions aggregate away from water.
Conclusion: Choosing Hydrophilic or Nonpolar Materials
Choosing between hydrophilic and nonpolar materials depends on the intended application and environmental interaction requirements. Hydrophilic materials excel in applications requiring water absorption, adhesion, or biocompatibility, while nonpolar materials are preferred for water resistance, chemical stability, and low surface energy. Optimizing material selection involves evaluating factors such as polarity, solubility parameters, and surface tension to match performance with functional needs.
Hydrophilic Infographic
 libterm.com
libterm.com