Electron capture is a process in nuclear physics where an inner orbital electron is captured by the nucleus, combining with a proton to form a neutron and releasing a neutrino. This transformation decreases the atomic number by one while keeping the mass number unchanged, often leading to the emission of X-rays or Auger electrons. Explore the rest of the article to understand how electron capture influences radioactive decay and its applications in medicine and astrophysics.
Table of Comparison
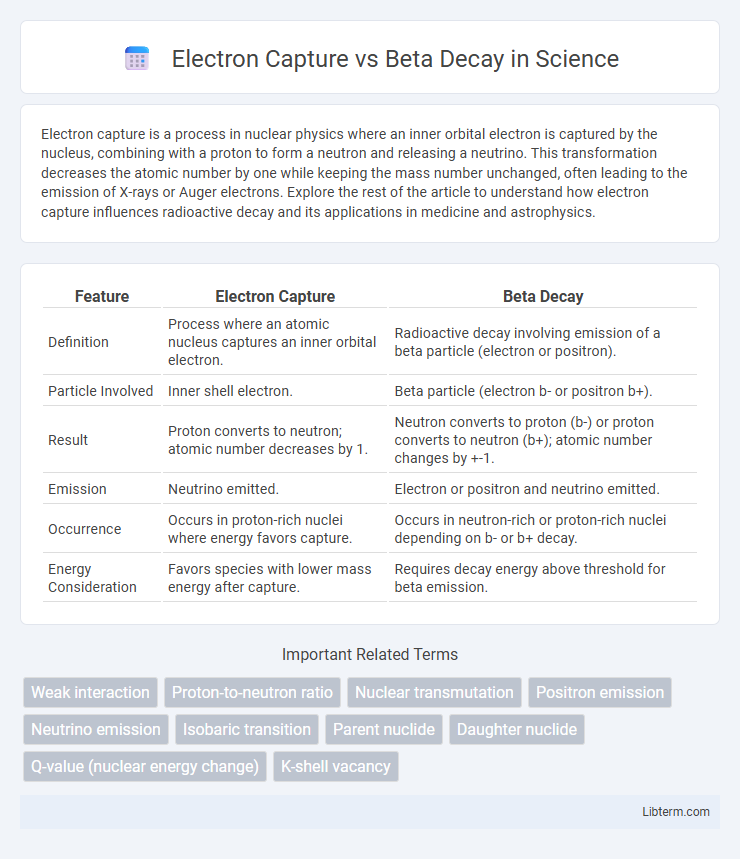
| Feature | Electron Capture | Beta Decay |
|---|---|---|
| Definition | Process where an atomic nucleus captures an inner orbital electron. | Radioactive decay involving emission of a beta particle (electron or positron). |
| Particle Involved | Inner shell electron. | Beta particle (electron b- or positron b+). |
| Result | Proton converts to neutron; atomic number decreases by 1. | Neutron converts to proton (b-) or proton converts to neutron (b+); atomic number changes by +-1. |
| Emission | Neutrino emitted. | Electron or positron and neutrino emitted. |
| Occurrence | Occurs in proton-rich nuclei where energy favors capture. | Occurs in neutron-rich or proton-rich nuclei depending on b- or b+ decay. |
| Energy Consideration | Favors species with lower mass energy after capture. | Requires decay energy above threshold for beta emission. |
Introduction to Nuclear Decay Processes
Electron capture involves the nucleus absorbing an inner orbital electron, leading to the conversion of a proton into a neutron and the emission of a neutrino. Beta decay occurs when a neutron transforms into a proton by emitting an electron (beta-minus decay) or a proton changes into a neutron emitting a positron (beta-plus decay), accompanied by neutrino or antineutrino emission. Both processes alter the atomic number of the nucleus, contributing to the transmutation of elements during nuclear decay.
What is Electron Capture?
Electron capture is a nuclear process in which an inner orbital electron is absorbed by the nucleus, combining with a proton to form a neutron and emitting a neutrino. This phenomenon typically occurs in proton-rich isotopes, decreasing the atomic number by one while leaving the mass number unchanged. Electron capture serves as an alternative to beta-plus decay in unstable isotopes seeking to achieve nuclear stability.
Understanding Beta Decay
Beta decay is a nuclear process in which a neutron transforms into a proton while emitting an electron (beta particle) and an antineutrino, altering the atomic number and leading to the formation of a different element. This decay occurs in neutron-rich nuclei to achieve greater stability by converting a neutron to a proton, thereby changing the isotopic identity. Beta decay contrasts with electron capture, where an inner orbital electron is absorbed by the nucleus, converting a proton into a neutron and emitting a neutrino.
Key Differences Between Electron Capture and Beta Decay
Electron capture involves a proton in the nucleus capturing an inner orbital electron and converting into a neutron, emitting a neutrino, whereas beta decay transforms a neutron into a proton while releasing an electron (beta particle) and an antineutrino. Electron capture decreases the atomic number by one without changing the mass number, while beta decay increases the atomic number by one, also keeping the mass number constant. The processes differ in emitted particles and nuclear changes, with electron capture common in proton-rich nuclei and beta decay occurring in neutron-rich nuclei.
Atomic Nucleus Changes: Electron Capture vs Beta Decay
Electron capture involves a proton in the atomic nucleus capturing an inner electron, converting the proton into a neutron and emitting a neutrino, which decreases the atomic number by one while the mass number remains unchanged. Beta decay occurs when a neutron in the nucleus transforms into a proton, emitting an electron (beta particle) and an antineutrino, thereby increasing the atomic number by one without altering the mass number. Both processes change the nucleus's proton-to-neutron ratio, affecting the element's identity but maintaining nuclear mass.
Conditions Favoring Electron Capture
Electron capture is favored in proton-rich nuclei where the energy released by converting a proton into a neutron is greater than the energy needed to emit a positron, making beta-plus decay energetically unfavorable. This process is more likely in atoms with higher atomic numbers because inner orbital electrons have a higher probability of being captured by the nucleus. High electron density around the nucleus and low decay energy thresholds significantly increase the likelihood of electron capture over beta decay.
Conditions Favoring Beta Decay
Beta decay is favored in nuclei with a neutron-to-proton ratio that is too high, allowing a neutron to transform into a proton while emitting an electron and an antineutrino. This process commonly occurs in isotopes far from the line of stability where the mass of the daughter nucleus is lower, releasing energy. High decay energy (Q-value) and a surplus of neutrons drive the nuclear reaction toward beta minus decay rather than electron capture.
Real-World Examples and Isotopes
Electron capture commonly occurs in proton-rich isotopes like potassium-40 and beryllium-7, where an inner orbital electron is captured by the nucleus, transforming a proton into a neutron. Beta decay is prevalent in neutron-rich isotopes such as carbon-14 and strontium-90, where a neutron decays into a proton while emitting a beta particle (electron or positron). Real-world applications include carbon-14 dating via beta decay and potassium-40 dating through electron capture, both critical for age determination in archaeology and geology.
Significance in Nuclear Medicine and Physics
Electron capture and beta decay are crucial nuclear processes with significant roles in nuclear medicine and physics. Electron capture is utilized in diagnostic imaging and radioisotope production due to its ability to create proton-rich isotopes, while beta decay is fundamental in radiotherapy and tracing radioactive isotopes in metabolic studies. Both processes provide insights into nuclear structure and help develop advanced medical imaging techniques like PET scans and targeted cancer treatments.
Summary: Choosing Between Electron Capture and Beta Decay
Electron capture occurs when a proton-rich nucleus absorbs an inner orbital electron, converting a proton into a neutron and emitting a neutrino, while beta decay involves the emission of an electron or positron to balance nuclear charge changes. The choice between electron capture and beta decay depends on the energy states and proton-to-neutron ratio within the nucleus, with electron capture favored in proton-rich isotopes where beta decay is energetically forbidden or less probable. Understanding these processes is crucial for predicting nuclear stability and decay pathways in isotopes like iodine-123 (electron capture) and carbon-14 (beta decay).
Electron Capture Infographic
 libterm.com
libterm.com