Acidic substances have a pH level below 7, indicating a higher concentration of hydrogen ions that can affect chemical reactions and biological processes. Understanding acidic properties is essential for managing soil health, food quality, and industrial applications. Explore the rest of the article to discover how acidic environments impact your daily life and practical tips for handling them effectively.
Table of Comparison
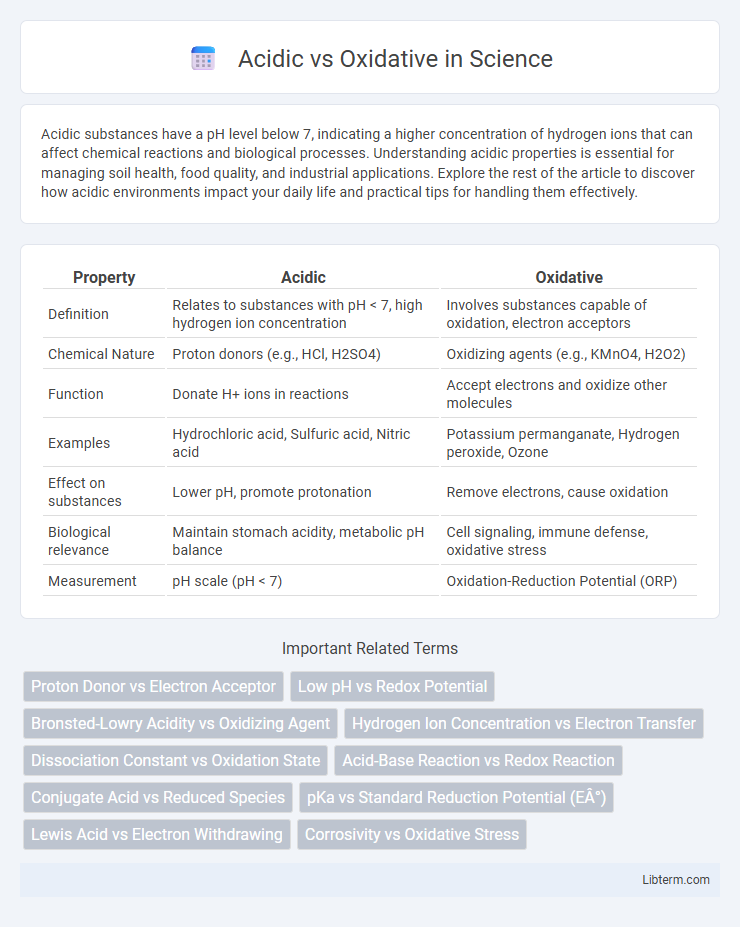
| Property | Acidic | Oxidative |
|---|---|---|
| Definition | Relates to substances with pH < 7, high hydrogen ion concentration | Involves substances capable of oxidation, electron acceptors |
| Chemical Nature | Proton donors (e.g., HCl, H2SO4) | Oxidizing agents (e.g., KMnO4, H2O2) |
| Function | Donate H+ ions in reactions | Accept electrons and oxidize other molecules |
| Examples | Hydrochloric acid, Sulfuric acid, Nitric acid | Potassium permanganate, Hydrogen peroxide, Ozone |
| Effect on substances | Lower pH, promote protonation | Remove electrons, cause oxidation |
| Biological relevance | Maintain stomach acidity, metabolic pH balance | Cell signaling, immune defense, oxidative stress |
| Measurement | pH scale (pH < 7) | Oxidation-Reduction Potential (ORP) |
Understanding Acidic and Oxidative Processes
Acidic processes involve the transfer of protons (H+ ions) leading to changes in pH and are essential in reactions like acid-base neutralization and catalysis. Oxidative processes involve the transfer of electrons, typically increasing the oxidation state of molecules, crucial in cellular respiration and combustion reactions. Understanding the molecular mechanisms of both acidic and oxidative processes helps optimize industrial chemical reactions and improve biochemical pathways.
Key Differences Between Acidity and Oxidation
Acidity refers to the ability of a substance to donate protons (H+ ions) or accept electron pairs, measured by pH or acid dissociation constant (Ka), while oxidation involves the loss of electrons from a molecule, atom, or ion, often assessed by changes in oxidation state. Acidic reactions primarily involve proton transfer and influence pH levels, whereas oxidative reactions involve electron transfer and often lead to the formation of new chemical bonds or compounds. Understanding acidity centers on Bronsted-Lowry and Lewis theories, whereas oxidation is central to redox chemistry and electron transfer processes.
Chemical Reactions: Acidic vs Oxidative Environments
Acidic environments promote chemical reactions involving proton (H+) transfer, often facilitating hydrolysis, esterification, and dissolution of metals by increasing ion availability. Oxidative environments drive reactions centered on electron transfer, typically resulting in oxidation-reduction processes that change oxidation states and generate reactive oxygen species. The distinction between acidic and oxidative reactions is critical in industrial applications such as corrosion control, organic synthesis, and environmental remediation.
Effects on Materials: Acidity vs Oxidative Damage
Acidic environments primarily cause corrosion and material degradation by breaking down metal surfaces and weakening structural integrity through proton attack. Oxidative damage involves the interaction with oxygen or other oxidizing agents, leading to rust formation, embrittlement, and loss of mechanical properties in metals. Materials exposed to oxidative conditions often experience surface oxidation layers, while acidic exposure results in more aggressive pitting and uniform corrosion.
Applications in Industry: Acidic and Oxidative Processes
Acidic processes are widely used in industries for metal refining, battery production, and chemical manufacturing due to their efficiency in dissolving materials and catalyzing reactions. Oxidative processes play a crucial role in wastewater treatment, chemical synthesis, and food preservation by promoting the breakdown of contaminants and enhancing product stability. Both acidic and oxidative techniques are integral to industries such as pharmaceuticals, water treatment, and metallurgy for optimizing performance and ensuring product quality.
Impacts on Human Health: Acidic vs Oxidative Exposure
Exposure to acidic substances can lead to respiratory irritation, skin burns, and eye damage due to their corrosive nature, significantly impacting human health by disrupting tissue integrity and causing inflammation. Oxidative agents generate free radicals that damage cellular components such as DNA, proteins, and lipids, contributing to chronic diseases like cancer, neurodegenerative disorders, and aging. Understanding the differential toxicological effects of acidic versus oxidative exposure is crucial for developing targeted prevention and treatment strategies in occupational and environmental health.
Environmental Consequences of Acidic and Oxidative Agents
Acidic agents contribute to environmental degradation by lowering pH levels in soil and water, leading to toxic conditions that harm aquatic life and reduce biodiversity. Oxidative agents cause oxidative stress in ecosystems, resulting in the breakdown of organic matter and cellular damage in plants and animals. Both acidification and oxidation disrupt ecological balance, causing long-term environmental consequences such as habitat loss and decreased ecosystem resilience.
Prevention and Mitigation Strategies
Preventing acidic and oxidative damage involves controlling environmental factors such as pH levels and exposure to reactive oxygen species (ROS). Implementing antioxidants like vitamin C and E, along with maintaining balanced dietary intake of alkaline foods, helps mitigate oxidative stress and acidity in tissues. Regular monitoring of industrial emissions and wastewater treatment reduces acidification and oxidative pollutants, protecting ecosystems and human health.
Testing and Measuring Acidity and Oxidation
Testing acidity involves measuring the pH level using indicators such as litmus paper, pH meters, or titration with a strong base to determine the concentration of hydrogen ions. Oxidation is assessed through redox potential measurements with electrodes or by analyzing reaction products using spectrophotometry and chemical assays to quantify electron transfer. Accurate acidity and oxidation measurements are essential for monitoring chemical reactions, environmental samples, and industrial processes.
Future Trends in Managing Acidic and Oxidative Processes
Future trends in managing acidic and oxidative processes emphasize the integration of advanced sensor technologies and AI-driven predictive analytics to optimize reaction control and minimize environmental impact. Development of green catalysts and sustainable reagents aims to enhance process efficiency while reducing harmful byproducts in industrial applications. Emerging biotechnological methods, including enzyme engineering and microbial consortia, offer promising solutions for environmentally friendly regulation of acidity and oxidation in both chemical manufacturing and waste treatment.
Acidic Infographic
 libterm.com
libterm.com