Enantiomeric compounds are molecules that are mirror images of each other but cannot be superimposed, playing a crucial role in chemistry and pharmacology due to their distinct biological activities. Understanding the differences between enantiomers can significantly impact drug development and the effectiveness of treatments. Explore the rest of the article to delve deeper into the fascinating world of enantiomeric interactions and their importance to your health and science.
Table of Comparison
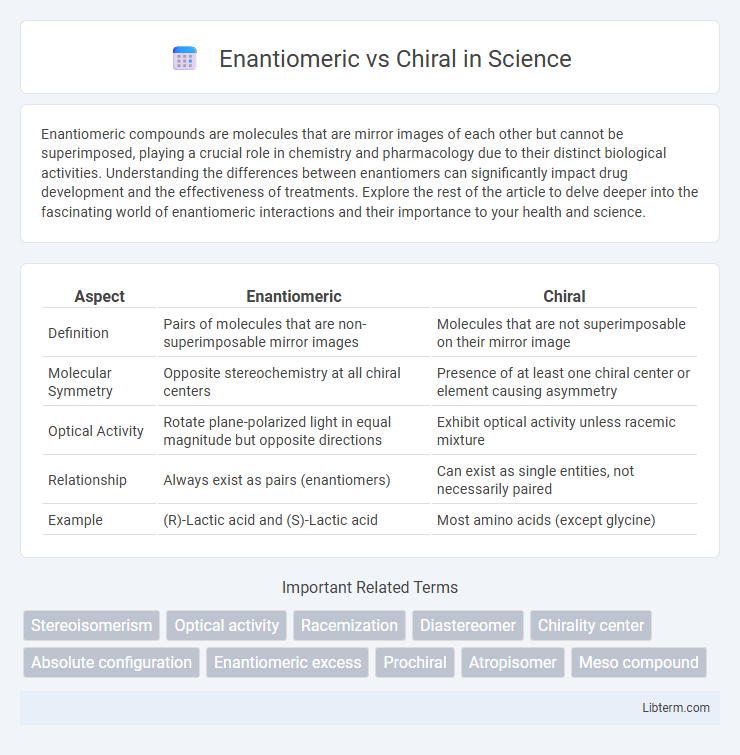
| Aspect | Enantiomeric | Chiral |
|---|---|---|
| Definition | Pairs of molecules that are non-superimposable mirror images | Molecules that are not superimposable on their mirror image |
| Molecular Symmetry | Opposite stereochemistry at all chiral centers | Presence of at least one chiral center or element causing asymmetry |
| Optical Activity | Rotate plane-polarized light in equal magnitude but opposite directions | Exhibit optical activity unless racemic mixture |
| Relationship | Always exist as pairs (enantiomers) | Can exist as single entities, not necessarily paired |
| Example | (R)-Lactic acid and (S)-Lactic acid | Most amino acids (except glycine) |
Introduction to Chirality and Enantiomerism
Chirality refers to the geometric property where an object or molecule is non-superimposable on its mirror image, often due to an asymmetric carbon atom. Enantiomers are pairs of chiral molecules that are mirror images but cannot be aligned perfectly, exhibiting identical physical properties except for their interaction with plane-polarized light and chiral environments. Understanding chirality and enantiomerism is crucial in stereochemistry, influencing biological activity, drug design, and molecular interactions.
Defining Chiral Molecules
Chiral molecules are defined by their non-superimposable mirror images, possessing an asymmetry that prevents overlap with their mirror counterparts. Unlike enantiomers, which are specific pairs of chiral molecules, chirality itself refers to the inherent geometric property of a molecule lacking an internal plane of symmetry. This spatial arrangement leads to distinct optical activities, critically impacting molecular interactions in biological and chemical systems.
What Are Enantiomers?
Enantiomers are pairs of molecules that are non-superimposable mirror images of each other, exhibiting chirality due to the presence of an asymmetric carbon atom. Each enantiomer has identical physical properties except for the direction in which they rotate plane-polarized light and their interactions with other chiral substances. Understanding enantiomers is crucial in fields like pharmaceuticals, where one enantiomer may have therapeutic effects while the other could be inactive or harmful.
Key Differences Between Chiral and Enantiomeric Compounds
Chiral compounds are molecules that have non-superimposable mirror images due to the presence of an asymmetric carbon atom, whereas enantiomers are pairs of chiral molecules that are mirror images of each other but not identical. The key difference lies in the fact that chirality refers to the molecular property, while enantiomers are specific stereoisomers exhibiting this chirality. Enantiomers have identical physical properties except for their interaction with plane-polarized light and reactions in chiral environments, which distinguishes them from other stereoisomers.
Importance of Stereochemistry in Chemistry
Enantiomers are pairs of chiral molecules that are non-superimposable mirror images, critically influencing drug efficacy and safety due to distinct biological activities. Chiral compounds possess at least one asymmetric carbon atom, causing stereoisomerism essential for molecular recognition in biochemical processes. Understanding stereochemistry enables chemists to design selective reactions and develop pharmaceuticals with targeted therapeutic effects.
Methods for Identifying Chiral Centers
Methods for identifying chiral centers primarily involve spectroscopic techniques such as circular dichroism (CD) and nuclear magnetic resonance (NMR) with chiral shift reagents, which differentiate enantiomers based on their interaction with polarized light or chiral environments. X-ray crystallography provides precise determination of absolute configuration by revealing the three-dimensional atomic arrangement in chiral molecules. Computational methods, including molecular modeling and quantum chemical calculations, predict stereochemistry and support experimental data for unambiguous chiral center identification.
How Enantiomers Affect Optical Activity
Enantiomers are pairs of chiral molecules that are non-superimposable mirror images, each rotating plane-polarized light in opposite directions, a phenomenon known as optical activity. The degree and direction of this optical rotation are specific to each enantiomer, allowing for the differentiation of stereoisomers in chiral compounds. Optical activity measurement is crucial in pharmaceuticals and stereochemistry for determining enantiomeric purity and pharmacological efficacy.
Applications of Chiral and Enantiomeric Substances
Chiral and enantiomeric substances find critical applications in pharmaceuticals, where the biological activity of enantiomers can differ drastically, necessitating enantiomerically pure drugs to enhance efficacy and reduce side effects. In agrochemicals, chiral compounds enable the development of selective pesticides and herbicides with improved environmental profiles and target specificity. Additionally, chiral catalysts are essential in asymmetric synthesis, facilitating the production of optically active compounds in chemical manufacturing and materials science.
Chiral vs. Enantiomeric: Common Misconceptions
Chiral refers to a molecule that is non-superimposable on its mirror image, whereas enantiomeric describes the relationship between two such chiral molecules that are mirror images of each other. A common misconception is that all chiral molecules are enantiomeric, but chiral compounds can exist without having an enantiomer in a given environment. Understanding this distinction is crucial for stereochemistry applications in pharmaceuticals and catalysis.
Future Trends in Chiral and Enantiomeric Research
Emerging trends in chiral and enantiomeric research emphasize the development of advanced asymmetric synthesis techniques and innovative chiral catalysts to enhance enantioselectivity in pharmaceutical applications. Cutting-edge analytical methods, such as chiral chromatography coupled with mass spectrometry, enable precise enantiomeric purity assessment and real-time monitoring during drug manufacturing. Advances in computational chemistry and machine learning are accelerating the prediction of chiral compound behavior, facilitating the design of safer and more effective enantiomerically pure drugs.
Enantiomeric Infographic
 libterm.com
libterm.com