Phosphorescence is the phenomenon where certain materials absorb energy and re-emit it as light over an extended period, even after the initial excitation source is removed. This prolonged glow results from trapped electrons slowly returning to their ground state, making it distinct from immediate fluorescence. Explore the rest of the article to discover how phosphorescence is applied in everyday life and advanced technologies.
Table of Comparison
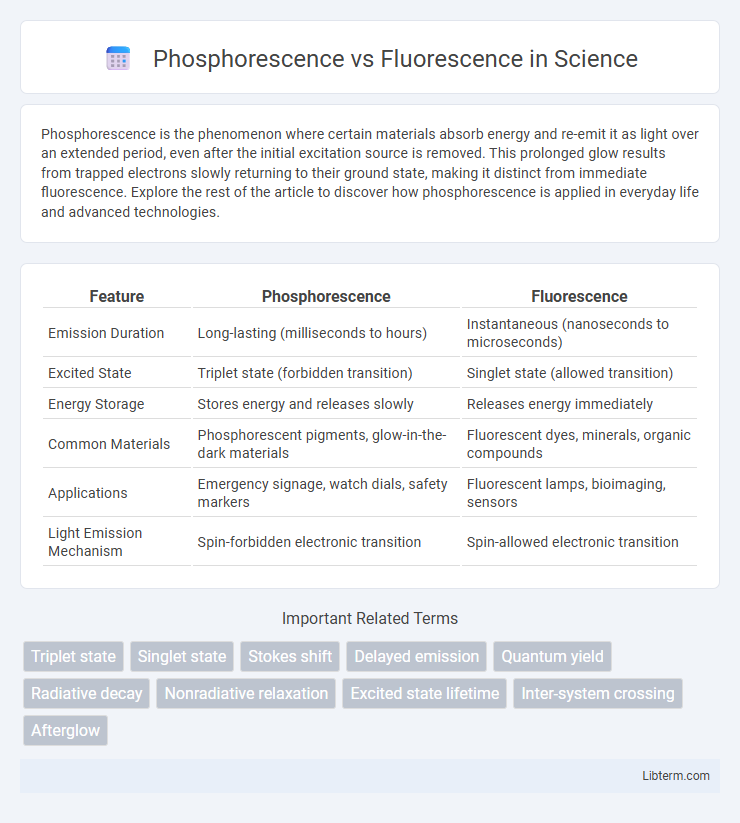
| Feature | Phosphorescence | Fluorescence |
|---|---|---|
| Emission Duration | Long-lasting (milliseconds to hours) | Instantaneous (nanoseconds to microseconds) |
| Excited State | Triplet state (forbidden transition) | Singlet state (allowed transition) |
| Energy Storage | Stores energy and releases slowly | Releases energy immediately |
| Common Materials | Phosphorescent pigments, glow-in-the-dark materials | Fluorescent dyes, minerals, organic compounds |
| Applications | Emergency signage, watch dials, safety markers | Fluorescent lamps, bioimaging, sensors |
| Light Emission Mechanism | Spin-forbidden electronic transition | Spin-allowed electronic transition |
Understanding Photoluminescence: Phosphorescence and Fluorescence
Phosphorescence and fluorescence are two types of photoluminescence where materials absorb photons and re-emit light at different timescales; fluorescence occurs within nanoseconds, while phosphorescence persists from microseconds to hours due to forbidden energy state transitions. The key difference lies in the electron spin states involved, with fluorescence resulting from singlet state transitions and phosphorescence involving triplet state transitions that delay photon emission. Understanding these mechanisms is crucial for applications in bioimaging, glow-in-the-dark materials, and optoelectronic devices where controlled light emission is essential.
Core Differences Between Phosphorescence and Fluorescence
Phosphorescence involves the prolonged emission of light due to the slow release of energy from triplet excited states, resulting in afterglow that can last from microseconds to hours. Fluorescence occurs when electrons quickly return from singlet excited states to the ground state, emitting light almost instantaneously, typically within nanoseconds to microseconds. The key difference lies in the spin states of the electrons and the duration of light emission, with phosphorescence exhibiting longer-lasting luminescence due to forbidden spin transitions.
Mechanisms of Light Emission
Phosphorescence occurs when absorbed energy excites electrons to a triplet state, causing delayed light emission as electrons return to the ground state over milliseconds to hours due to forbidden spin transitions. Fluorescence involves electrons excited to a singlet state, emitting light almost instantly--within nanoseconds--when returning to the ground state through allowed spin transitions. The fundamental difference in spin state transitions accounts for the varying durations and intensities between phosphorescence and fluorescence light emission mechanisms.
Electron Excitation and Relaxation Pathways
Phosphorescence involves electron excitation to a triplet excited state, followed by a slow intersystem crossing and a delayed relaxation to the ground singlet state, resulting in prolonged light emission. Fluorescence occurs when electrons excite to a singlet excited state and quickly relax back to the ground state, emitting light almost instantaneously. The key difference lies in the spin state transitions and relaxation times: phosphorescence features forbidden spin transitions causing extended emission, while fluorescence entails allowed singlet-singlet transitions with rapid decay.
Lifespan of Emission: Immediate vs Delayed Glow
Phosphorescence exhibits a delayed glow due to its extended emission lifespan, often lasting from milliseconds to several hours after the excitation source is removed. Fluorescence produces an immediate emission with a lifespan typically in the nanosecond to microsecond range, ceasing almost instantly once the excitation light is turned off. This fundamental difference in emission duration results from the distinct electron spin states involved, with phosphorescence involving forbidden triplet-to-singlet transitions that slow down photon release.
Common Applications in Industry and Science
Phosphorescence is widely used in safety signage, watch dials, and glow-in-the-dark materials due to its long-lasting afterglow, enabling visibility in low-light conditions. Fluorescence finds applications in bioimaging, medical diagnostics, and chemical sensing, leveraging its rapid emission response to ultraviolet or visible light for detecting specific molecules. Both processes are integral to industrial quality control and scientific research, with fluorescence aiding in real-time analysis and phosphorescence enhancing durability in luminescent products.
Material Examples: Phosphorescent vs Fluorescent Compounds
Phosphorescent materials include zinc sulfide doped with copper and strontium aluminate, known for their long-lasting afterglow used in glow-in-the-dark products. Fluorescent compounds commonly consist of fluorescein and rhodamine dyes, which emit light almost immediately when exposed to ultraviolet or visible light sources, making them ideal for applications in bioimaging and fluorescent lamps. The key difference lies in the electron relaxation time, with phosphorescent materials exhibiting delayed emission due to forbidden energy state transitions, unlike the rapid fluorescence emission.
Role in Biological Imaging and Detection
Phosphorescence and fluorescence are critical in biological imaging and detection, with fluorescence enabling real-time visualization of cellular processes due to its rapid emission decay times. Phosphorescence, characterized by longer emission lifetimes, facilitates time-gated imaging techniques that reduce background noise and improve signal specificity in bioimaging applications. Fluorescent dyes and phosphorescent probes are extensively utilized in techniques such as fluorescence microscopy and phosphorescence lifetime imaging microscopy (PLIM) to enhance contrast and detect biomolecular interactions effectively.
Environmental and Energy Considerations
Phosphorescence offers longer light emission durations, making it advantageous for energy-saving applications such as low-energy lighting and nighttime visibility without continuous power input. Fluorescence, with its rapid emission decay, is effective in applications requiring immediate light response but generally consumes more energy due to the need for constant excitation. Environmentally, phosphorescent materials reduce energy consumption and carbon footprint by minimizing electrical usage, whereas fluorescent materials' demand for constant energy input can lead to higher environmental impacts if not efficiently managed.
Future Trends in Luminescent Technologies
Phosphorescence and fluorescence are key phenomena in luminescent technologies, with phosphorescence offering prolonged afterglow and fluorescence providing rapid light emission. Future trends emphasize the development of organic and quantum dot materials to enhance efficiency, stability, and tunability of these emissions for applications in flexible displays, bio-imaging, and security features. Advances in nanostructured phosphors and time-resolved fluorescence techniques are expected to drive innovations in next-generation lighting and sensing devices.
Phosphorescence Infographic
 libterm.com
libterm.com