Meso refers to the middle layer in various scientific and technical contexts, such as mesosphere in atmospheric science or mesoporous materials in chemistry. Understanding meso-scale phenomena is crucial for innovations in fields like climate studies, materials science, and urban planning. Discover how meso concepts impact your field and explore detailed insights in the rest of this article.
Table of Comparison
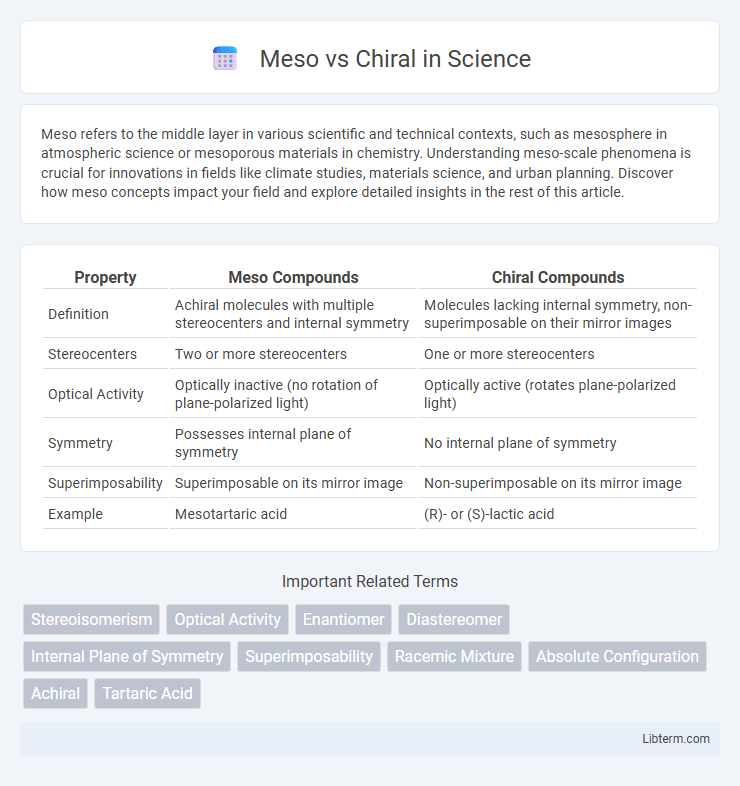
| Property | Meso Compounds | Chiral Compounds |
|---|---|---|
| Definition | Achiral molecules with multiple stereocenters and internal symmetry | Molecules lacking internal symmetry, non-superimposable on their mirror images |
| Stereocenters | Two or more stereocenters | One or more stereocenters |
| Optical Activity | Optically inactive (no rotation of plane-polarized light) | Optically active (rotates plane-polarized light) |
| Symmetry | Possesses internal plane of symmetry | No internal plane of symmetry |
| Superimposability | Superimposable on its mirror image | Non-superimposable on its mirror image |
| Example | Mesotartaric acid | (R)- or (S)-lactic acid |
Introduction to Meso and Chiral Compounds
Meso compounds possess multiple stereocenters but are achiral due to an internal plane of symmetry that renders them optically inactive. Chiral compounds lack this internal symmetry, resulting in non-superimposable mirror images known as enantiomers, which exhibit optical activity. Understanding the structural differences between meso and chiral compounds is crucial for stereochemistry, impacting their chemical behavior and biological interactions.
Defining Chirality: Key Concepts
Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image, a fundamental concept in stereochemistry. Meso compounds are unique molecules that contain multiple stereocenters but exhibit internal symmetry, resulting in achirality despite having chiral centers. This distinction highlights the importance of spatial arrangement and symmetry elements in determining the overall chirality of a molecule.
What Are Meso Compounds?
Meso compounds are achiral molecules that contain multiple stereocenters but possess an internal plane of symmetry, rendering them optically inactive despite having chiral centers. These compounds exhibit superimposable mirror images due to their symmetrical configuration, distinguishing them from chiral molecules that lack such symmetry and are non-superimposable. Understanding meso compounds is essential in stereochemistry because they influence the optical activity and enantiomeric purity of synthetic reactions and pharmaceutical compounds.
Structural Criteria for Meso Compounds
Meso compounds are characterized by the presence of multiple stereocenters and an internal plane of symmetry, rendering them achiral despite having chiral centers. The structural criteria for meso compounds require that substituents on stereocenters are arranged in such a way that one half of the molecule is a mirror image of the other half. Unlike chiral molecules, which lack any symmetry element connecting stereocenters, meso compounds have this symmetry element that cancels optical activity.
Chirality Centers: Identification and Examples
Meso compounds contain multiple chirality centers but are achiral due to an internal plane of symmetry, which cancels optical activity. Chiral molecules, on the other hand, lack such symmetry and have non-superimposable mirror images, often leading to enantiomers. For example, tartaric acid exhibits both meso and chiral forms: the meso form has two stereocenters with a plane of symmetry, while its enantiomeric pair lacks this symmetry and is optically active.
Symmetry in Meso vs Chiral Molecules
Meso molecules possess internal planes of symmetry that make them achiral despite having multiple stereocenters, resulting in optical inactivity. In contrast, chiral molecules lack any internal symmetry, leading to non-superimposable mirror images and optical activity. The presence or absence of symmetry elements is the key factor distinguishing meso compounds from chiral compounds.
Stereochemical Properties of Meso and Chiral Compounds
Meso compounds possess multiple stereocenters but are achiral due to an internal plane of symmetry, resulting in optical inactivity despite the presence of stereogenic centers. Chiral compounds lack such symmetry, causing non-superimposability on their mirror images and exhibiting optical activity by rotating plane-polarized light. The stereochemical distinction between meso and chiral compounds significantly impacts their behavior in enantiomeric excess and resolution techniques in stereochemistry.
Optical Activity: Differences Between Meso and Chiral
Meso compounds are optically inactive despite containing stereocenters because they possess an internal plane of symmetry that causes their optical rotations to cancel out. Chiral molecules lack this plane of symmetry, resulting in non-superimposable mirror images and exhibiting optical activity by rotating plane-polarized light. The key difference in optical activity between meso and chiral substances lies in their molecular symmetry, which governs whether or not they can rotate polarized light.
Real-World Examples: Meso vs Chiral Compounds
Meso compounds like tartaric acid exhibit internal symmetry, rendering them achiral despite multiple stereocenters, which is crucial in pharmaceuticals for predictable bioactivity. Chiral compounds such as (R)-limonene possess non-superimposable mirror images, leading to distinct smells and interactions in drug design and fragrance industries. Understanding the differences between meso and chiral molecules aids in stereochemical control during synthesis, impacting efficacy and safety in real-world applications.
Significance in Organic Synthesis and Applications
Meso compounds, characterized by internal planes of symmetry, exhibit unique stereochemical properties that make them crucial in asymmetric synthesis as they often serve as achiral intermediates or ligands, minimizing racemic mixtures. Chiral molecules, lacking such symmetry, are essential in creating enantiomerically pure products, which are vital in pharmaceuticals where stereochemistry significantly affects drug efficacy and safety. Both meso and chiral compounds play pivotal roles in enantioselective catalysis and stereoselective transformations, driving advancements in organic synthesis and industrial applications.
Meso Infographic
 libterm.com
libterm.com