Diffusion is the process by which molecules move from an area of higher concentration to one of lower concentration, seeking equilibrium. This fundamental mechanism plays a crucial role in various biological, chemical, and physical systems, facilitating the transport of substances across membranes and within environments. Explore the full article to understand how diffusion impacts your everyday life and scientific phenomena.
Table of Comparison
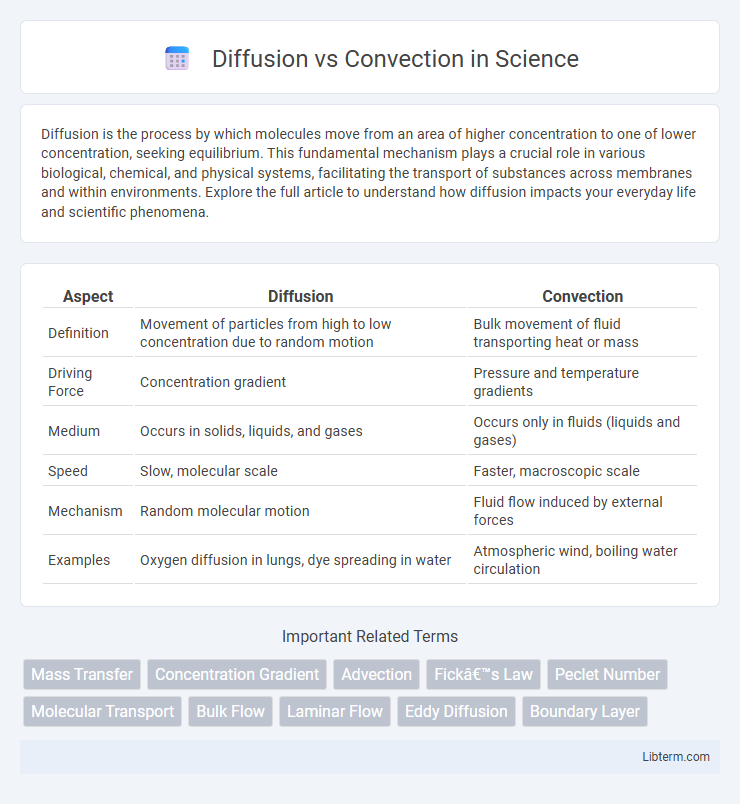
| Aspect | Diffusion | Convection |
|---|---|---|
| Definition | Movement of particles from high to low concentration due to random motion | Bulk movement of fluid transporting heat or mass |
| Driving Force | Concentration gradient | Pressure and temperature gradients |
| Medium | Occurs in solids, liquids, and gases | Occurs only in fluids (liquids and gases) |
| Speed | Slow, molecular scale | Faster, macroscopic scale |
| Mechanism | Random molecular motion | Fluid flow induced by external forces |
| Examples | Oxygen diffusion in lungs, dye spreading in water | Atmospheric wind, boiling water circulation |
Understanding Diffusion: Definition and Mechanism
Diffusion is the passive movement of molecules from an area of higher concentration to an area of lower concentration, driven by the concentration gradient. This process occurs due to the random thermal motion of particles and does not require external energy. Diffusion plays a crucial role in processes such as gas exchange in lungs and nutrient transport across cell membranes.
What Is Convection? An Overview
Convection is the transfer of heat through the movement of fluids, such as liquids or gases, driven by temperature differences causing fluid motion. It involves the bulk movement of molecules within the fluid, facilitating energy transfer more efficiently than diffusion alone. This process plays a crucial role in natural phenomena and engineering applications, including atmospheric circulation, ocean currents, and heat exchangers.
Key Differences Between Diffusion and Convection
Diffusion is the passive movement of particles from an area of higher concentration to lower concentration driven by concentration gradients, whereas convection involves the bulk movement of fluid carrying substances along with it, influenced by external forces such as gravity or mechanical stirring. Diffusion occurs at the molecular level without energy input, while convection requires energy to induce fluid motion, significantly increasing the rate of heat or mass transfer. Key differences include the mechanisms involved, dependence on concentration gradients versus fluid velocity, and the scale at which transport occurs.
Molecular Motion: How Diffusion Works
Diffusion relies on the random thermal motion of molecules, causing them to move from regions of higher concentration to lower concentration until equilibrium is reached. Molecular collisions and Brownian motion drive this passive transport without requiring external energy or bulk fluid movement. This process is critical in gases and liquids for mixing and mass transfer at the microscopic scale.
The Role of Fluid Flow in Convection
Fluid flow in convection enhances heat and mass transfer by moving fluid particles, creating temperature and concentration gradients that accelerate energy distribution. Unlike diffusion, which relies on molecular motion alone, convection utilizes bulk fluid movement to increase the rate of transport significantly. This dynamic process is crucial in natural phenomena such as atmospheric circulation and industrial applications like heat exchangers.
Factors Affecting Diffusion Rates
Diffusion rates are primarily influenced by concentration gradients, temperature, and the medium through which particles move, with higher temperatures increasing kinetic energy and thus accelerating diffusion. Molecular size and the viscosity of the medium also play critical roles, as smaller molecules diffuse faster and lower viscosity facilitates easier particle movement. Unlike convection, which depends on bulk fluid motion driven by pressure or temperature gradients, diffusion relies solely on random molecular motion, making these factors crucial in determining diffusion efficiency.
Factors Influencing Convection Efficiency
Convection efficiency is influenced primarily by fluid velocity, temperature gradient, and fluid properties such as viscosity and thermal conductivity. Increased fluid velocity enhances heat transfer rates through turbulent mixing, while a steeper temperature gradient drives stronger buoyancy forces in natural convection. Surface geometry and orientation also affect convection by altering flow patterns and boundary layer development, directly impacting the overall heat transfer coefficient.
Real-World Examples of Diffusion
Diffusion occurs naturally in processes such as the exchange of oxygen and carbon dioxide in the lungs, where gases move from regions of higher concentration to lower concentration without external forces. In wastewater treatment, diffusion enables pollutants to spread into microbial communities, facilitating biodegradation. Another example includes the gradual mixing of perfume molecules in air, demonstrating diffusion-driven mass transport at the molecular level.
Practical Applications of Convection Processes
Convection processes play a crucial role in practical applications such as heating, ventilation, and air conditioning (HVAC) systems, where the forced movement of air ensures efficient temperature regulation in buildings. Industrial operations utilize convection for cooling electronic components and in chemical reactors to maintain uniform temperature distribution. Natural convection is also essential in meteorology, influencing weather patterns and ocean currents by transferring heat through fluid motion.
Diffusion vs Convection: Summary Table and Key Takeaways
Diffusion involves the passive movement of particles from regions of high concentration to low concentration, driven by concentration gradients, whereas convection entails the bulk movement of fluid carrying particles due to external forces like pressure or temperature differences. The summary table highlights diffusion's reliance on molecular motion without energy input, contrasting with convection's dependence on fluid dynamics and external energy. Key takeaways emphasize diffusion's role in microscale transport and slow processes, while convection dominates macroscale and rapid transport phenomena in engineering and environmental systems.
Diffusion Infographic
 libterm.com
libterm.com