Apoptotic processes are essential for maintaining cellular health by systematically eliminating damaged or unnecessary cells through programmed cell death. Understanding how apoptotic mechanisms function can reveal potential therapeutic targets for diseases like cancer, where cell death regulation is disrupted. Explore this article further to gain deeper insights into how apoptotic pathways impact your overall health and treatment options.
Table of Comparison
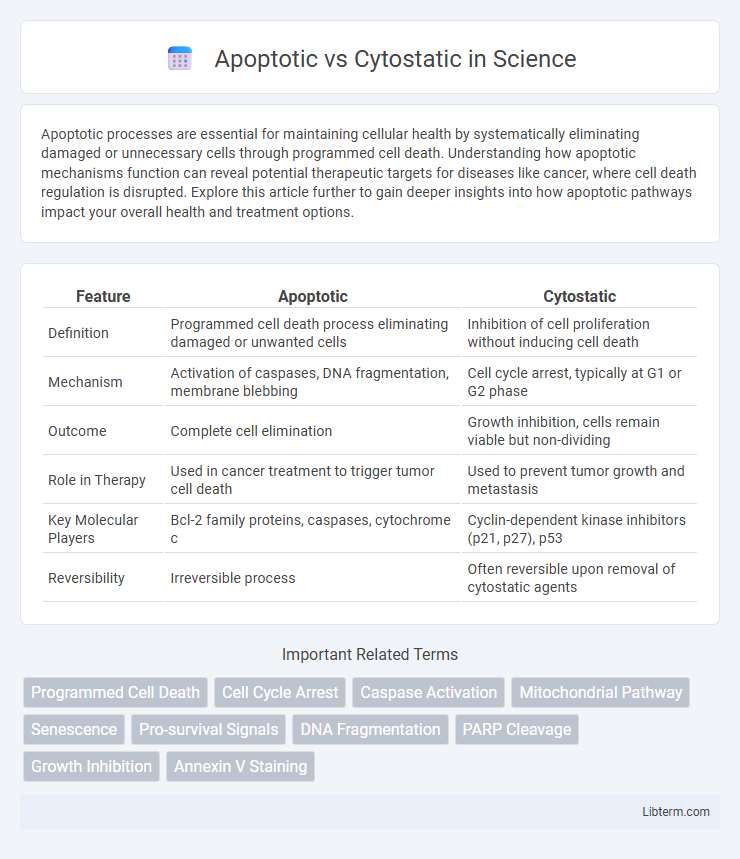
| Feature | Apoptotic | Cytostatic |
|---|---|---|
| Definition | Programmed cell death process eliminating damaged or unwanted cells | Inhibition of cell proliferation without inducing cell death |
| Mechanism | Activation of caspases, DNA fragmentation, membrane blebbing | Cell cycle arrest, typically at G1 or G2 phase |
| Outcome | Complete cell elimination | Growth inhibition, cells remain viable but non-dividing |
| Role in Therapy | Used in cancer treatment to trigger tumor cell death | Used to prevent tumor growth and metastasis |
| Key Molecular Players | Bcl-2 family proteins, caspases, cytochrome c | Cyclin-dependent kinase inhibitors (p21, p27), p53 |
| Reversibility | Irreversible process | Often reversible upon removal of cytostatic agents |
Introduction to Apoptotic and Cytostatic Mechanisms
Apoptotic mechanisms trigger programmed cell death through a tightly regulated cascade involving caspases and mitochondrial pathways to eliminate damaged or unwanted cells. Cytostatic mechanisms inhibit cell proliferation by arresting the cell cycle, often through modulation of cyclin-dependent kinases (CDKs) and checkpoint activation to prevent tumorigenesis. Understanding these distinct cellular processes is crucial for developing targeted therapies in cancer treatment.
Defining Apoptosis: Programmed Cell Death
Apoptosis is a tightly regulated process of programmed cell death essential for maintaining cellular homeostasis and eliminating damaged or harmful cells without triggering inflammation. Unlike cytostatic effects that merely halt cell proliferation, apoptosis involves characteristic morphological changes such as chromatin condensation, membrane blebbing, and formation of apoptotic bodies. Key molecular pathways include activation of caspases, mitochondrial outer membrane permeabilization, and regulation by Bcl-2 family proteins, ensuring a controlled cellular dismantling crucial for tissue development and immune system function.
Understanding Cytostasis: Halting Cell Growth
Cytostasis refers to the reversible inhibition of cell growth and proliferation without inducing cell death, contrasting with apoptosis, which is the programmed elimination of cells. Understanding cytostasis involves analyzing signaling pathways that regulate the cell cycle, such as cyclin-dependent kinase inhibitors (e.g., p21, p27), which enforce cell cycle arrest at checkpoints like G1 or G2/M phases. Targeting cytostatic mechanisms is crucial in cancer therapy to suppress tumor progression by maintaining cells in a dormant state while minimizing cytotoxic side effects.
Molecular Pathways: Apoptotic vs Cytostatic Signals
Apoptotic signals activate molecular pathways involving caspases, Bcl-2 family proteins, and mitochondrial outer membrane permeabilization leading to programmed cell death. Cytostatic signals primarily inhibit cell cycle progression through cyclin-dependent kinase inhibitors like p21 and p27 or modulation of retinoblastoma protein (Rb) activity, causing growth arrest without inducing apoptosis. These distinct pathways determine whether a cell undergoes apoptosis or remains in a reversible quiescent state, impacting therapeutic strategies targeting cancer cell proliferation.
Cellular Outcomes: Death vs Dormancy
Apoptotic mechanisms trigger programmed cell death characterized by DNA fragmentation, membrane blebbing, and phagocytic clearance, leading to irreversible elimination of damaged or harmful cells. Cytostatic effects induce cellular dormancy by arresting the cell cycle, halting proliferation without initiating cell death pathways, which preserves cell viability and metabolic activity. The key distinction lies in apoptosis causing cell clearance and population reduction, while cytostasis maintains cell survival in a non-dividing, quiescent state.
Key Regulators in Apoptosis and Cytostasis
Key regulators in apoptosis include caspases, Bcl-2 family proteins, and p53, which orchestrate cell death by promoting mitochondrial outer membrane permeabilization and DNA fragmentation. In cytostasis, cyclin-dependent kinase inhibitors such as p21, p27, and retinoblastoma protein enforce cell cycle arrest by halting the progression from G1 to S phase, preventing cellular proliferation without inducing cell death. Both apoptosis and cytostasis are tightly controlled by signaling pathways that respond to cellular stress and damage, ensuring tissue homeostasis and preventing oncogenic transformation.
Clinical Implications in Cancer Therapy
Apoptotic therapies induce programmed cell death, effectively eliminating cancer cells and reducing tumor burden, crucial for overcoming resistance in aggressive cancers. Cytostatic treatments primarily halt tumor proliferation by arresting cell cycle progression, which can improve disease management but may require combination with apoptosis-inducing agents for complete remission. Understanding the balance between apoptotic and cytostatic mechanisms enables personalized cancer therapy strategies, optimizing efficacy while minimizing adverse effects.
Drug Development: Targeting Apoptotic and Cytostatic Pathways
Targeting apoptotic pathways in drug development involves designing agents that induce programmed cell death, which is crucial for eliminating cancerous or diseased cells. Cytostatic drugs focus on halting cell proliferation by arresting the cell cycle, preventing tumor growth without necessarily triggering apoptosis. Combining apoptotic and cytostatic mechanisms enhances therapeutic efficacy, offering precise control over cell fate in cancer treatment and reducing resistance to conventional therapies.
Resistance Mechanisms: Evasion of Cell Death and Growth Arrest
Resistance mechanisms in apoptotic pathways often involve the upregulation of anti-apoptotic proteins such as Bcl-2 and IAPs, which inhibit caspase activation and prevent programmed cell death. Cytostatic resistance typically arises from alterations in cell cycle regulators like p21 and p27, enabling cells to bypass growth arrest signals and continue proliferating despite therapeutic intervention. Both evasion of apoptosis and bypass of cytostatic arrest contribute to tumor survival and treatment failure by disrupting controlled cell death and growth inhibition processes.
Future Perspectives in Apoptotic and Cytostatic Research
Emerging therapies targeting apoptotic pathways hold promise for precision oncology by selectively inducing cancer cell death through modulation of BCL-2 family proteins and caspases. Cytostatic agents, which inhibit cell proliferation without triggering apoptosis, are being optimized for synergistic use with immunotherapies to enhance tumor suppression while minimizing toxicity. Advances in single-cell sequencing and high-throughput screening platforms accelerate identification of biomarkers, facilitating personalized treatment strategies that combine apoptotic and cytostatic mechanisms for improved clinical outcomes.
Apoptotic Infographic
 libterm.com
libterm.com