Adiabatic processes involve changes in a system where no heat is transferred to or from the environment, resulting in temperature changes due solely to work done on or by the system. This principle is fundamental in thermodynamics, impacting fields like meteorology, engineering, and atmospheric sciences. Explore the rest of the article to understand how adiabatic processes influence energy efficiency and natural phenomena.
Table of Comparison
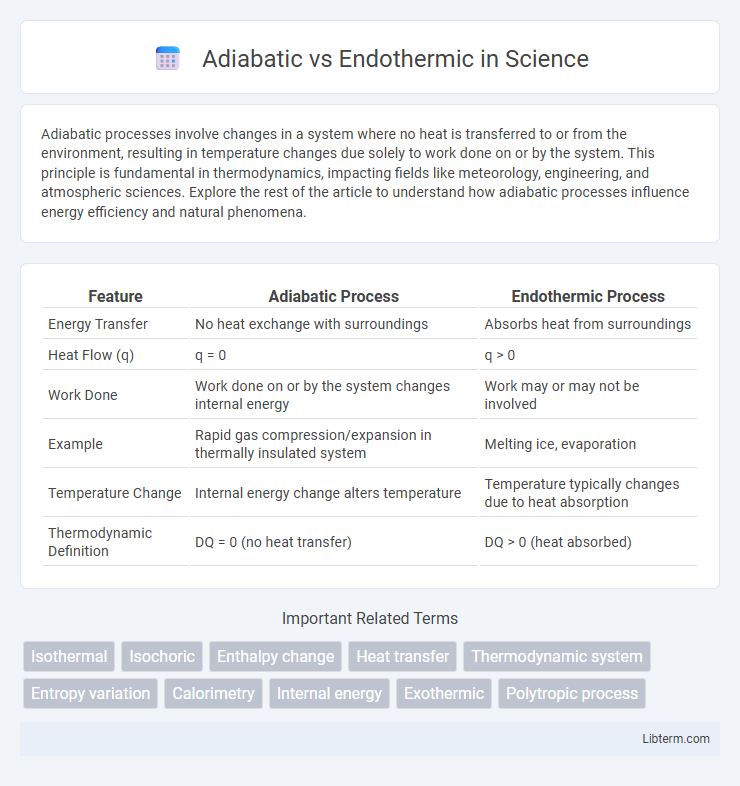
| Feature | Adiabatic Process | Endothermic Process |
|---|---|---|
| Energy Transfer | No heat exchange with surroundings | Absorbs heat from surroundings |
| Heat Flow (q) | q = 0 | q > 0 |
| Work Done | Work done on or by the system changes internal energy | Work may or may not be involved |
| Example | Rapid gas compression/expansion in thermally insulated system | Melting ice, evaporation |
| Temperature Change | Internal energy change alters temperature | Temperature typically changes due to heat absorption |
| Thermodynamic Definition | DQ = 0 (no heat transfer) | DQ > 0 (heat absorbed) |
Understanding Adiabatic and Endothermic Processes
Adiabatic processes involve no heat exchange with the surroundings, meaning all energy transfer occurs as work, resulting in changes in internal energy without heat flow. Endothermic processes require the absorption of heat from the environment to proceed, often causing a temperature decrease in the surroundings. Understanding the distinction between adiabatic and endothermic processes is crucial for analyzing thermodynamic systems, as it impacts how energy and heat transfer influence system behavior.
Key Differences Between Adiabatic and Endothermic Reactions
Adiabatic reactions occur without heat exchange with the surroundings, maintaining constant internal energy, while endothermic reactions absorb heat from their environment, causing a net energy increase in the system. In adiabatic processes, temperature changes result solely from work done on or by the system, whereas endothermic reactions rely on external heat input to proceed. The key difference lies in heat transfer: adiabatic reactions are thermally isolated, endothermic reactions require continuous heat absorption.
Thermodynamic Principles Behind Each Process
Adiabatic processes occur without heat exchange between the system and its surroundings, relying solely on work done to change the system's internal energy, following the first law of thermodynamics. Endothermic processes absorb heat from the surroundings, leading to an increase in the system's internal energy, and are characterized by positive enthalpy changes (DH > 0). The key thermodynamic distinction lies in energy transfer modes: adiabatic processes involve no heat transfer (Q=0), whereas endothermic processes require heat input (Q>0) to drive phase changes or chemical reactions.
Energy Exchange: Isolated vs. Heat Absorbing Systems
Adiabatic processes involve no heat exchange with the surroundings, resulting in energy transfer solely through work within an isolated system. Endothermic reactions require the absorption of heat energy from the environment, causing an increase in the system's internal energy. Understanding the distinction between adiabatic and endothermic processes is crucial for analyzing thermodynamic systems regarding energy exchange and system isolation.
Practical Examples of Adiabatic Processes
Adiabatic processes occur without heat exchange between a system and its surroundings, as seen in the rapid compression of gases within a diesel engine cylinder where temperature rises due to work done on the gas. Another practical example includes the expansion of air parcels in the atmosphere, which cools adiabatically during ascent, contributing to cloud formation and weather patterns. These processes contrast with endothermic reactions that require continuous heat absorption, such as the melting of ice or photosynthesis in plants.
Common Endothermic Reactions in Daily Life
Endothermic reactions absorb heat from their surroundings, causing temperature drops noticeable in daily life examples like ice melting, photosynthesis in plants, and evaporation of sweat for cooling. These processes require continuous energy intake to proceed, unlike adiabatic reactions where no heat exchange occurs with the environment. Common endothermic reactions emphasize the critical role of energy absorption in natural and physiological functions, highlighting their importance in temperature regulation and chemical synthesis.
Real-world Applications in Industry and Science
Adiabatic processes are pivotal in thermodynamic systems such as gas turbines and refrigeration cycles, where no heat exchange with the environment occurs, optimizing energy efficiency and system performance. Endothermic reactions, absorbing heat, are fundamental in chemical manufacturing processes like the synthesis of ammonia via the Haber process and in materials engineering for heat treatment techniques. Both concepts underpin crucial innovations in energy conversion, environmental control, and industrial chemical reactions, driving advancements in sustainable technology and manufacturing efficiency.
Comparing Temperature Changes in Both Processes
Adiabatic processes involve temperature changes due to compression or expansion without heat exchange with the surroundings, resulting in a temperature increase or decrease solely from work done on or by the system. Endothermic processes absorb heat from the surroundings, causing the temperature of the system to decrease as energy is taken in to break chemical bonds or induce phase changes. Comparing both, adiabatic temperature changes depend on internal energy variations from work, while endothermic temperature changes arise from external heat absorption.
Importance in Chemical and Physical Systems
Adiabatic processes involve no heat exchange with the surroundings, making them critical in systems where insulation is essential, such as in thermodynamic cycles and engines. Endothermic reactions absorb heat, playing a vital role in chemical processes like photosynthesis and industrial synthesis where energy input drives transformations. Understanding the differences ensures precise control over energy flow and efficiency in both physical and chemical systems.
Summary Table: Adiabatic vs. Endothermic at a Glance
The Summary Table: Adiabatic vs. Endothermic at a Glance highlights key differences, showing adiabatic processes occur without heat exchange with the surroundings, while endothermic reactions absorb heat from the environment. Adiabatic changes involve temperature variations solely due to work done on or by the system, whereas endothermic reactions require external energy input to proceed. This comparison aids in understanding thermodynamic and chemical system behaviors under different energy transfer conditions.
Adiabatic Infographic
 libterm.com
libterm.com