Catabolic processes break down complex molecules into simpler ones, releasing energy essential for cellular activities and maintaining metabolic balance. These reactions play a crucial role in energy production, especially during periods of fasting or intense physical activity. Discover how catabolic pathways impact Your body's energy management and overall health in the rest of this article.
Table of Comparison
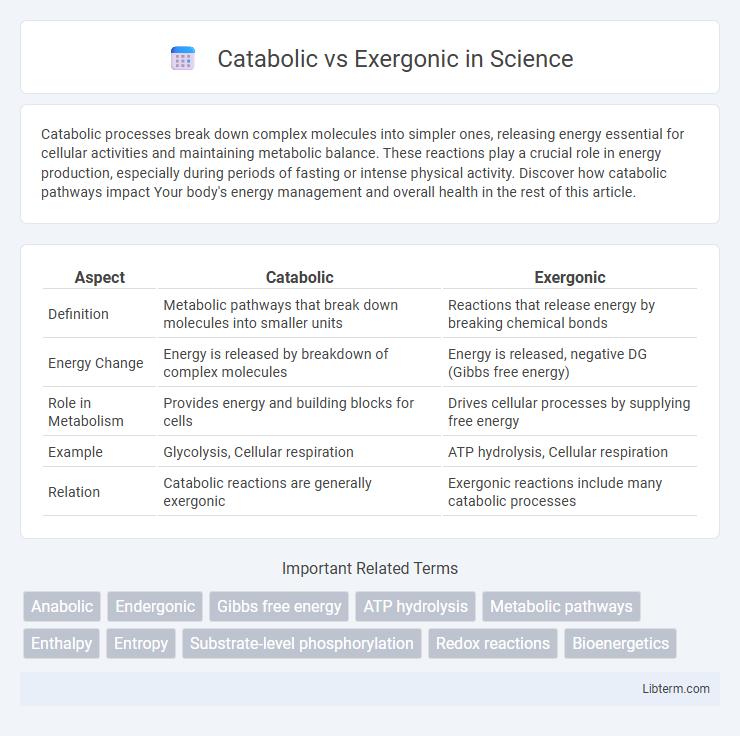
| Aspect | Catabolic | Exergonic |
|---|---|---|
| Definition | Metabolic pathways that break down molecules into smaller units | Reactions that release energy by breaking chemical bonds |
| Energy Change | Energy is released by breakdown of complex molecules | Energy is released, negative DG (Gibbs free energy) |
| Role in Metabolism | Provides energy and building blocks for cells | Drives cellular processes by supplying free energy |
| Example | Glycolysis, Cellular respiration | ATP hydrolysis, Cellular respiration |
| Relation | Catabolic reactions are generally exergonic | Exergonic reactions include many catabolic processes |
Introduction to Catabolic and Exergonic Processes
Catabolic processes involve the breakdown of complex molecules into simpler ones, releasing energy essential for cellular functions. Exergonic reactions, characterized by a negative Gibbs free energy change, release energy as they proceed and drive these catabolic activities. Understanding the distinction between catabolic pathways and exergonic reaction mechanisms is crucial for studying metabolic energy flow.
Defining Catabolic Reactions
Catabolic reactions are metabolic processes that break down complex molecules into simpler ones, releasing energy stored in chemical bonds. These reactions are typically exergonic, meaning they release free energy that cells harness for biological functions. Key examples of catabolic processes include glycolysis and the citric acid cycle, which degrade glucose and other macromolecules to produce ATP.
Understanding Exergonic Reactions
Exergonic reactions release energy by breaking chemical bonds, making the process spontaneous and energetically favorable. These reactions often occur in catabolic pathways where complex molecules such as glucose are broken down into simpler compounds, releasing ATP. The free energy change (DG) in exergonic reactions is negative, reflecting the net release of energy available for cellular work.
Key Differences Between Catabolic and Exergonic
Catabolic processes involve the breakdown of complex molecules into simpler ones, releasing energy stored in chemical bonds, while exergonic reactions specifically describe any chemical reaction that releases free energy, making both concepts closely related but distinct in scope. Key differences include that catabolic pathways are a subset of metabolic processes aimed at energy release through molecule degradation, whereas exergonic reactions encompass a broader range of energy-releasing chemical reactions beyond metabolism. Catabolic reactions are typically exergonic, but not all exergonic reactions are catabolic, as some exergonic reactions may occur without molecular breakdown.
Examples of Catabolic Pathways
Catabolic pathways break down complex molecules into simpler ones, releasing energy often captured as ATP. Examples include glycolysis, where glucose is converted into pyruvate, and the citric acid cycle, which oxidizes acetyl-CoA to CO2 while generating NADH and FADH2. Another key example is beta-oxidation, the process of fatty acid degradation producing acetyl-CoA for energy production.
Examples of Exergonic Reactions
Exergonic reactions release energy by breaking down molecules, with common examples including cellular respiration, where glucose is oxidized to produce ATP, and the hydrolysis of ATP into ADP and inorganic phosphate, which powers numerous cellular processes. Another key example is the combustion of glucose in mitochondria, yielding carbon dioxide, water, and energy essential for biological functions. These reactions are characterized by a negative Gibbs free energy change, indicating spontaneity and energy release during the process.
Role of Enzymes in Catabolic and Exergonic Processes
Enzymes play a crucial role in catabolic and exergonic processes by accelerating the breakdown of complex molecules into simpler ones, releasing energy stored in chemical bonds. In catabolic pathways, enzymes such as hydrolases and oxidoreductases facilitate reactions that degrade substrates, leading to energy release essential for cellular functions. During exergonic reactions, enzymes lower activation energy, ensuring efficient energy transfer that drives metabolic activities like ATP synthesis and heat production.
Biological Significance of Catabolic vs Exergonic
Catabolic reactions involve the breakdown of complex molecules into simpler ones, releasing energy essential for cellular functions. Exergonic reactions are characterized by a negative Gibbs free energy change, making them spontaneously favorable, often driving metabolic processes forward. The biological significance of catabolic reactions lies in their role as primary sources of energy, while exergonic reactions provide the thermodynamic basis that powers these energy-releasing pathways.
Catabolic and Exergonic Reactions in Metabolism
Catabolic reactions break down complex molecules into simpler ones, releasing energy stored in chemical bonds. Exergonic reactions occur when these catabolic processes release more energy than they consume, making them spontaneous and energetically favorable. In metabolism, catabolic and exergonic reactions are crucial for generating ATP, which powers cellular activities.
Summary: Catabolic vs Exergonic in Biochemistry
Catabolic reactions involve the breakdown of complex molecules into simpler ones, releasing energy stored in chemical bonds. Exergonic reactions are characterized by a negative Gibbs free energy change (DG < 0), indicating that they release energy to the surroundings during the process. While all catabolic reactions are exergonic because they release energy, not all exergonic reactions are catabolic, as some may involve processes other than molecular breakdown.
Catabolic Infographic
 libterm.com
libterm.com