Efficient cooling systems play a crucial role in maintaining optimal temperatures for comfort and equipment performance. Proper cooling solutions help prevent overheating, reduce energy consumption, and extend the lifespan of your devices. Explore the full article to discover the best cooling strategies tailored to your needs.
Table of Comparison
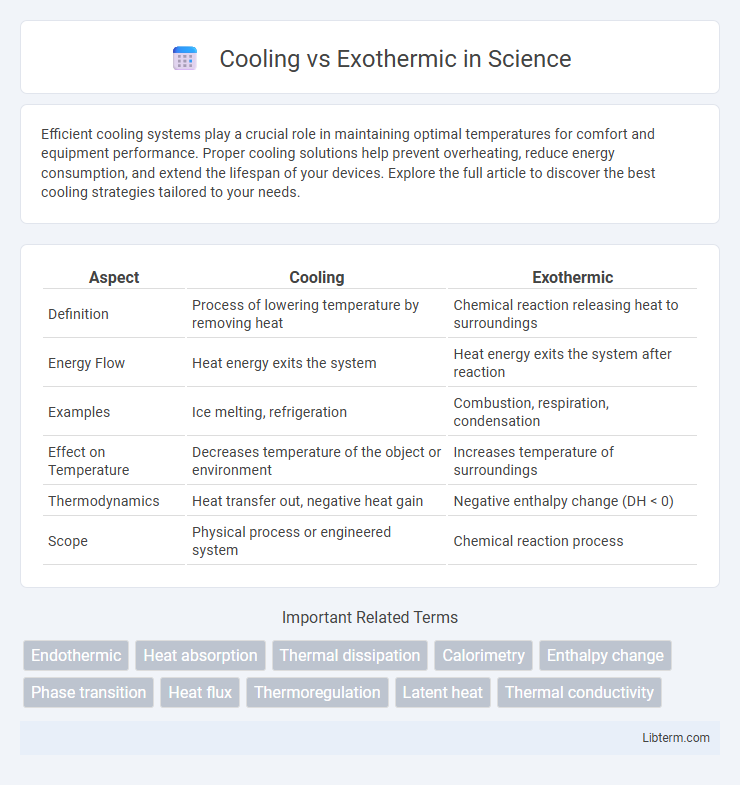
| Aspect | Cooling | Exothermic |
|---|---|---|
| Definition | Process of lowering temperature by removing heat | Chemical reaction releasing heat to surroundings |
| Energy Flow | Heat energy exits the system | Heat energy exits the system after reaction |
| Examples | Ice melting, refrigeration | Combustion, respiration, condensation |
| Effect on Temperature | Decreases temperature of the object or environment | Increases temperature of surroundings |
| Thermodynamics | Heat transfer out, negative heat gain | Negative enthalpy change (DH < 0) |
| Scope | Physical process or engineered system | Chemical reaction process |
Understanding Cooling and Exothermic Processes
Cooling processes involve the transfer of heat from a substance to its surroundings, resulting in a decrease in the substance's temperature and thermal energy. Exothermic processes release energy, usually in the form of heat, as a chemical reaction or physical change occurs, increasing the temperature of the surroundings. Understanding the distinction between cooling and exothermic reactions is essential for controlling energy flow in scientific and industrial applications.
Key Differences Between Cooling and Exothermic Reactions
Cooling refers to the process of reducing temperature by removing heat from a system, while exothermic reactions release heat as a product of chemical changes. Key differences include that cooling is a physical process involving heat transfer without chemical transformation, whereas exothermic reactions involve chemical bonds breaking and forming to emit heat. Cooling decreases the thermal energy of a substance, whereas exothermic reactions increase the surrounding environment's temperature by releasing stored chemical energy.
Science Behind Cooling Mechanisms
Cooling mechanisms involve the absorption or removal of heat, often through endothermic processes where energy is taken from the environment, lowering the temperature. Exothermic reactions release heat energy, increasing the surrounding temperature by converting chemical energy into thermal energy. Understanding the thermodynamic principles behind heat transfer and molecular interactions is essential for optimizing cooling technologies and controlling exothermic reactions in industrial applications.
How Exothermic Reactions Release Heat
Exothermic reactions release heat by forming new chemical bonds that have lower energy than the bonds in the reactants, causing excess energy to be emitted as heat. During these reactions, the energy difference between reactants and products is transferred to the surroundings, increasing the ambient temperature. Cooling processes, in contrast, absorb heat to reduce system temperature, thus acting opposite to heat-releasing exothermic reactions.
Common Examples of Cooling in Everyday Life
Cooling processes involve the removal of heat from a substance, often resulting in temperature reduction, while exothermic reactions release heat during chemical changes. Common examples of cooling in everyday life include refrigeration, where heat is extracted from food to preserve it, air conditioning systems that cool indoor environments by expelling heat, and evaporative cooling such as sweating, which lowers body temperature through heat loss during evaporation. These examples highlight practical applications of cooling mechanisms in daily human activities.
Everyday Instances of Exothermic Reactions
Exothermic reactions release heat, making them common in everyday activities such as combustion in car engines and the oxidation of iron during rusting. Cooling is the process of heat removal, often observed when sweating helps regulate body temperature by dissipating heat from exothermic metabolic reactions. Understanding exothermic reactions in daily life highlights their role in energy transfer and temperature changes within natural and industrial environments.
Industrial Applications: Cooling vs Exothermic Methods
Industrial applications utilize cooling methods such as refrigeration and heat exchangers to efficiently remove excess heat and maintain optimal process temperatures, ensuring equipment longevity and product quality. Exothermic methods exploit heat generated from chemical reactions, like in cement curing or polymerization, to reduce external energy input and enhance energy efficiency. Selecting between cooling and exothermic approaches depends on specific process requirements, thermal management needs, and energy consumption goals within industries like chemical manufacturing and metal processing.
Safety Considerations in Cooling and Exothermic Processes
Cooling processes require careful monitoring to prevent thermal shock and ensure safe temperature gradients, especially in chemical and industrial systems. Exothermic reactions pose risks of rapid temperature rises and potential runaway reactions, necessitating robust heat dissipation methods and emergency shutdown protocols. Proper safety measures, including temperature sensors, cooling jackets, and controlled reaction rates, are essential to mitigate hazards in both cooling and exothermic processes.
Environmental Impact: Cooling vs Exothermic Activities
Cooling activities, such as air conditioning and refrigeration, consume significant amounts of electricity, often derived from fossil fuels, leading to increased greenhouse gas emissions and exacerbating climate change. Exothermic reactions release heat, which can contribute to environmental warming if not properly managed, and some industrial exothermic processes emit pollutants or toxic byproducts. Efficient energy use and emission controls in both cooling systems and exothermic processes are crucial for minimizing their negative environmental impacts.
Choosing the Right Method: Cooling or Exothermic Approach
Choosing between a cooling or exothermic approach depends on the specific chemical reaction and desired outcome. Cooling methods effectively control reaction rates and prevent overheating in exothermic processes, ensuring safety and stability. An exothermic approach can be advantageous when the reaction's heat release drives further transformation or reduces external energy input.
Cooling Infographic
 libterm.com
libterm.com