Oxidation is a chemical reaction where a substance loses electrons, often involving oxygen, altering the material's properties and structure. This process is critical in various fields, from biology, where it impacts cellular functions, to industry, affecting metal corrosion and aging. Discover how understanding oxidation can help you manage its effects and protect your materials by reading the rest of this article.
Table of Comparison
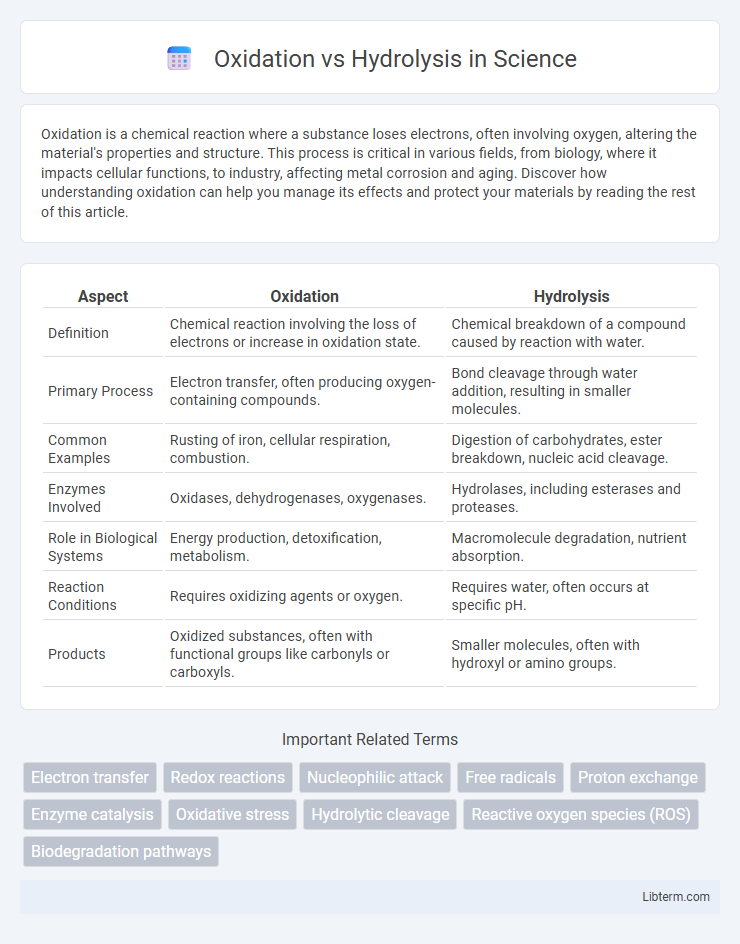
| Aspect | Oxidation | Hydrolysis |
|---|---|---|
| Definition | Chemical reaction involving the loss of electrons or increase in oxidation state. | Chemical breakdown of a compound caused by reaction with water. |
| Primary Process | Electron transfer, often producing oxygen-containing compounds. | Bond cleavage through water addition, resulting in smaller molecules. |
| Common Examples | Rusting of iron, cellular respiration, combustion. | Digestion of carbohydrates, ester breakdown, nucleic acid cleavage. |
| Enzymes Involved | Oxidases, dehydrogenases, oxygenases. | Hydrolases, including esterases and proteases. |
| Role in Biological Systems | Energy production, detoxification, metabolism. | Macromolecule degradation, nutrient absorption. |
| Reaction Conditions | Requires oxidizing agents or oxygen. | Requires water, often occurs at specific pH. |
| Products | Oxidized substances, often with functional groups like carbonyls or carboxyls. | Smaller molecules, often with hydroxyl or amino groups. |
Introduction to Oxidation and Hydrolysis
Oxidation involves the chemical reaction where a substance loses electrons, often reacting with oxygen, leading to the formation of oxides or other oxidized compounds. Hydrolysis is a chemical process that breaks bonds in molecules through the reaction with water, commonly affecting esters, amides, and carbohydrates. Both oxidation and hydrolysis play crucial roles in biochemical pathways and industrial applications such as metabolism and polymer degradation.
Defining Oxidation: Process and Mechanisms
Oxidation is a chemical process involving the loss of electrons from a molecule, atom, or ion, often resulting in an increase in oxidation state. This mechanism commonly involves the reaction with oxygen or other oxidizing agents, facilitating electron transfer and the formation of new chemical bonds. Key examples include the oxidation of metals, organic compounds, and the cellular respiration processes critical for energy production.
Understanding Hydrolysis: Key Concepts and Reactions
Hydrolysis involves the chemical breakdown of a compound due to reaction with water, leading to the cleavage of bonds such as esters, amides, or glycosidic linkages. Key reactions include the hydrolysis of triglycerides into glycerol and fatty acids, catalyzed by lipase enzymes, and the digestion of carbohydrates via amylase-mediated hydrolysis producing simple sugars. Understanding the role of water as a nucleophile and factors like pH and enzyme presence is essential in predicting hydrolysis reaction outcomes.
Chemical Differences: Oxidation vs Hydrolysis
Oxidation involves the loss of electrons or an increase in oxidation state of a molecule, often resulting in the addition of oxygen or removal of hydrogen, while hydrolysis is a chemical reaction where water breaks down compounds by cleaving bonds, typically between two molecules or within a polymer. Oxidation reactions commonly alter the electron configuration and introduce oxygen-containing functional groups such as carbonyls or peroxides. Hydrolysis specifically targets bond cleavage, producing smaller molecules like acids, alcohols, or amines through nucleophilic attack by water molecules on susceptible bonds such as esters, amides, or glycosidic linkages.
Common Examples in Daily Life
Rust formation on iron surfaces exemplifies oxidation, where iron reacts with oxygen and moisture, causing corrosion. Hydrolysis occurs when baking soda reacts with vinegar, producing carbon dioxide gas as a result of the decomposition of sodium bicarbonate in the presence of water. Both oxidation and hydrolysis are essential chemical reactions influencing everyday phenomena such as food spoilage, metal degradation, and cooking processes.
Industrial Applications and Importance
Oxidation processes are crucial in industries such as chemical manufacturing, refining, and waste treatment, enabling the conversion of raw materials into valuable products like acids, alcohols, and polymers. Hydrolysis is extensively applied in the production of biofuels, pharmaceuticals, and detergents by breaking down complex molecules into simpler components for enhanced reactivity and usability. Both reactions are fundamental for industrial efficiency, environmental sustainability, and the development of advanced materials.
Impact on Food and Pharmaceutical Stability
Oxidation leads to the degradation of food and pharmaceutical compounds by causing rancidity, nutrient loss, and reduced shelf life through the reaction with oxygen, prominently affecting fats and vitamins. Hydrolysis breaks chemical bonds in molecules like esters and peptides, resulting in altered drug efficacy, texture changes in foods, and increased susceptibility to microbial growth. Both processes compromise product stability, necessitating protective measures such as antioxidants and moisture control to preserve quality and safety.
Environmental and Biological Implications
Oxidation processes significantly impact environmental systems by contributing to the degradation of pollutants and the formation of reactive oxygen species that affect aquatic and atmospheric chemistry. Hydrolysis plays a crucial role in biological systems by breaking down complex molecules such as proteins, carbohydrates, and pesticides, facilitating nutrient cycling and detoxification in soil and water. Both oxidation and hydrolysis are essential in biogeochemical cycles, influencing ecosystem health through pollutant transformation and nutrient availability.
How to Prevent Oxidation and Hydrolysis
To prevent oxidation, store products in airtight containers away from light and heat, and use antioxidants like vitamin E or ascorbic acid to slow the reaction. Hydrolysis prevention involves controlling moisture levels by keeping items dry, avoiding exposure to water, and using desiccants or moisture barriers in packaging. Both processes require minimizing exposure to environmental factors such as oxygen, humidity, and temperature to extend product stability and shelf life.
Conclusion: Choosing the Right Reaction Pathway
Oxidation is ideal for applications requiring electron transfer and energy production, often involving oxygen or other oxidizing agents, which makes it crucial in metabolism and industrial synthesis. Hydrolysis excels in breaking chemical bonds through water addition, playing a key role in digestion, polymer degradation, and chemical recycling processes. Selecting the right reaction pathway depends on the desired chemical transformation, substrate specificity, and environmental conditions, with oxidation preferred for redox modifications and hydrolysis favored for bond cleavage and molecule breakdown.
Oxidation Infographic
 libterm.com
libterm.com