Aqueous solutions play a crucial role in various chemical and biological processes due to their ability to dissolve many substances effectively. Understanding the properties and behaviors of aqueous environments can enhance your approach to scientific experiments and industrial applications. Explore the rest of the article to learn how aqueous systems impact everyday life and advanced technologies.
Table of Comparison
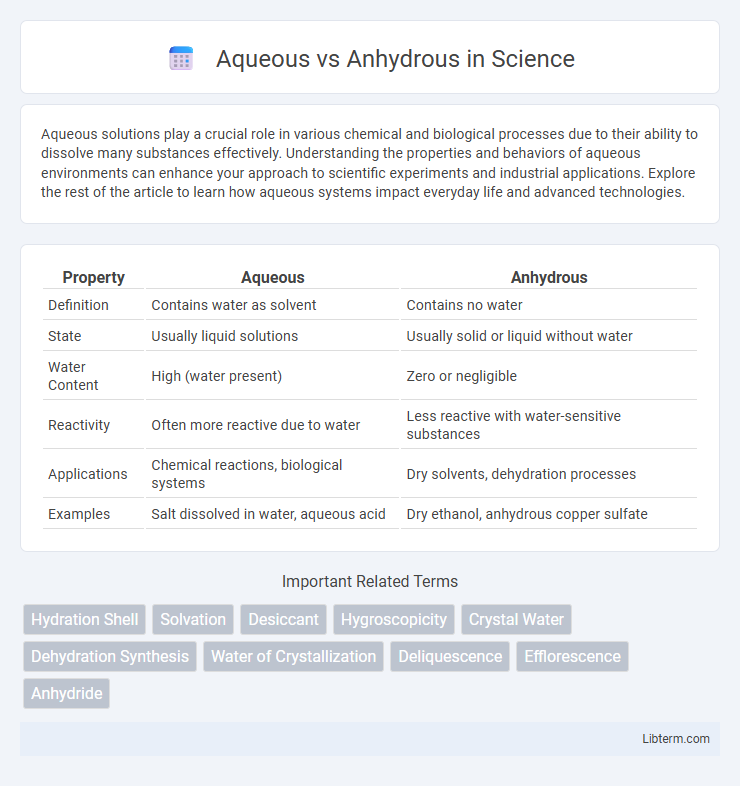
| Property | Aqueous | Anhydrous |
|---|---|---|
| Definition | Contains water as solvent | Contains no water |
| State | Usually liquid solutions | Usually solid or liquid without water |
| Water Content | High (water present) | Zero or negligible |
| Reactivity | Often more reactive due to water | Less reactive with water-sensitive substances |
| Applications | Chemical reactions, biological systems | Dry solvents, dehydration processes |
| Examples | Salt dissolved in water, aqueous acid | Dry ethanol, anhydrous copper sulfate |
Introduction to Aqueous and Anhydrous Systems
Aqueous systems involve substances dissolved in water, creating solutions essential for various chemical reactions and biological processes due to water's polar nature and solvent capabilities. Anhydrous systems lack water, often used in chemical synthesis where moisture can interfere with reactions or reduce product purity. Understanding the differences between aqueous and anhydrous environments is crucial for selecting appropriate conditions in laboratory experiments and industrial applications.
Defining Aqueous Solutions
Aqueous solutions consist of substances dissolved in water, forming a homogeneous mixture where water acts as the solvent. These solutions are essential in various chemical reactions, as water facilitates ionization and molecular interactions. Understanding the properties of aqueous solutions is crucial in fields like chemistry, biology, and environmental science.
Understanding Anhydrous Compounds
Anhydrous compounds are chemical substances that contain no water molecules within their crystalline structure, distinguishing them from aqueous solutions where water acts as a solvent. These compounds often exhibit different physical and chemical properties such as melting points, solubility, and reactivity due to the absence of water. Understanding the stability and behavior of anhydrous compounds is crucial in fields like pharmaceuticals and materials science, where moisture sensitivity impacts formulation and storage.
Key Differences: Aqueous vs Anhydrous
Aqueous solutions contain water as the solvent, facilitating ionization and conductivity in chemical reactions, whereas anhydrous substances lack water, often resulting in different physical properties and reactivity. The presence of water in aqueous solutions influences solubility, reaction rates, and equilibrium dynamics, while anhydrous forms are essential in processes requiring moisture-free environments, such as in certain synthesis or storage. Understanding these key differences is critical for applications in chemistry, pharmaceuticals, and industrial processes where moisture content affects performance and stability.
Common Examples of Aqueous Solutions
Aqueous solutions commonly include saltwater, where sodium chloride dissolves in water, and sugar solutions, such as glucose or sucrose dissolved in water, used extensively in food and pharmaceutical industries. Industrial processes frequently utilize aqueous hydrochloric acid and sulfuric acid solutions for cleaning and chemical synthesis due to their high reactivity in water. In contrast, anhydrous substances, like anhydrous copper sulfate or ethanol, lack water, affecting their chemical properties and applications significantly.
Common Examples of Anhydrous Compounds
Anhydrous compounds, such as anhydrous calcium chloride, sodium sulfate, and copper(II) sulfate, are widely used in industrial and laboratory settings for their moisture-absorbing properties. These substances are free of water molecules, unlike their aqueous counterparts, which contain water as a solvent or within their crystal structure. Common anhydrous materials serve critical roles in drying agents, chemical synthesis, and creating controlled reaction environments.
Chemical Properties and Reactivity
Aqueous solutions contain water as a solvent, enabling ionic compounds to dissociate and enhancing reactivity through solvation and proton transfer mechanisms, whereas anhydrous substances lack water, often exhibiting higher reactivity due to the absence of solvation effects and stronger intermolecular interactions. In aqueous environments, hydrolysis and acid-base reactions proceed readily, while anhydrous conditions favor direct interactions between reactants, often leading to different reaction pathways or product distributions. The presence or absence of water significantly influences reaction kinetics, equilibrium constants, and the stability of intermediates in chemical processes.
Applications in Industry and Laboratories
Aqueous solutions are widely used in pharmaceutical formulations, chemical synthesis, and biochemical assays due to their excellent solubility and ease of handling, enabling efficient reactions and processes in laboratories and industrial settings. Anhydrous substances play a critical role in industries such as electronics manufacturing and organometallic chemistry, where moisture-sensitive reagents require strictly controlled, water-free environments to maintain stability and reaction specificity. Both forms are essential in analytical chemistry, with aqueous mediums facilitating titrations and spectrophotometric analyses, while anhydrous conditions are crucial for processes like gas-phase reactions and solvent-free catalytic transformations.
Safety and Handling Considerations
Aqueous solutions generally require careful handling due to their potential for corrosion, chemical reactivity, and spill hazards, necessitating appropriate personal protective equipment such as gloves and goggles. Anhydrous chemicals, being water-free, often exhibit higher reactivity and flammability, demanding stringent storage conditions, moisture-free environments, and specific inert atmospheres to prevent hazardous reactions. Proper labeling, ventilation, and emergency protocols are critical for both forms to ensure safe usage and minimize risks in laboratory or industrial settings.
Choosing Between Aqueous and Anhydrous
Choosing between aqueous and anhydrous forms depends on the chemical reaction and desired outcomes, as aqueous solutions involve water as a solvent, facilitating ionization and faster reaction rates. Anhydrous conditions eliminate water to prevent hydrolysis or unwanted side reactions, often essential in moisture-sensitive syntheses and catalytic processes. The decision hinges on factors such as solubility, reactivity, and product stability to optimize efficiency and yield in industrial or laboratory applications.
Aqueous Infographic
 libterm.com
libterm.com