Gluconeogenesis is the metabolic process by which your body generates glucose from non-carbohydrate sources, essential during fasting or intense exercise. This pathway primarily occurs in the liver and helps maintain stable blood sugar levels when dietary glucose is scarce. Discover how gluconeogenesis impacts your energy balance and overall health by reading the full article.
Table of Comparison
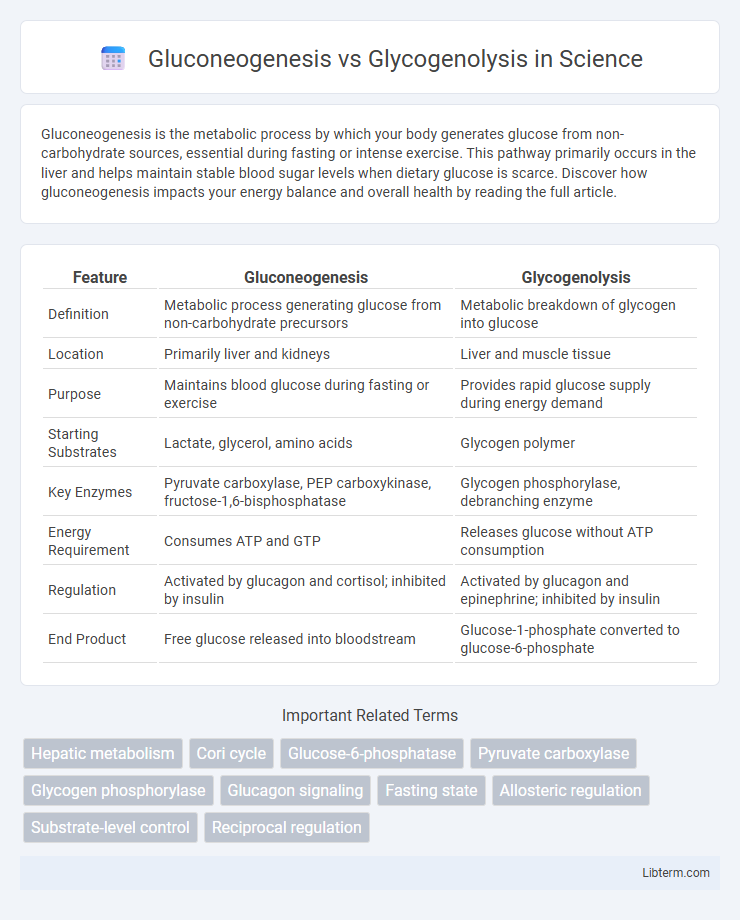
| Feature | Gluconeogenesis | Glycogenolysis |
|---|---|---|
| Definition | Metabolic process generating glucose from non-carbohydrate precursors | Metabolic breakdown of glycogen into glucose |
| Location | Primarily liver and kidneys | Liver and muscle tissue |
| Purpose | Maintains blood glucose during fasting or exercise | Provides rapid glucose supply during energy demand |
| Starting Substrates | Lactate, glycerol, amino acids | Glycogen polymer |
| Key Enzymes | Pyruvate carboxylase, PEP carboxykinase, fructose-1,6-bisphosphatase | Glycogen phosphorylase, debranching enzyme |
| Energy Requirement | Consumes ATP and GTP | Releases glucose without ATP consumption |
| Regulation | Activated by glucagon and cortisol; inhibited by insulin | Activated by glucagon and epinephrine; inhibited by insulin |
| End Product | Free glucose released into bloodstream | Glucose-1-phosphate converted to glucose-6-phosphate |
Introduction to Gluconeogenesis and Glycogenolysis
Gluconeogenesis is the metabolic pathway that synthesizes glucose from non-carbohydrate sources such as lactate, glycerol, and amino acids, primarily occurring in the liver and kidneys during fasting or intense exercise. Glycogenolysis involves the breakdown of glycogen stores into glucose-1-phosphate, rapidly providing glucose to maintain blood sugar levels during short-term energy demands. Both processes are crucial for maintaining glucose homeostasis but differ in their substrates and regulatory mechanisms.
Defining Gluconeogenesis: Pathways and Key Enzymes
Gluconeogenesis is the metabolic pathway that generates glucose from non-carbohydrate substrates such as lactate, glycerol, and amino acids, primarily occurring in the liver and kidneys. Key enzymes involved include pyruvate carboxylase, which converts pyruvate to oxaloacetate, phosphoenolpyruvate carboxykinase (PEPCK) that facilitates the formation of phosphoenolpyruvate, and fructose-1,6-bisphosphatase, responsible for bypassing the irreversible glycolytic step catalyzed by phosphofructokinase. This pathway contrasts with glycogenolysis, which involves the breakdown of glycogen stores into glucose-1-phosphate, catalyzed by glycogen phosphorylase, serving as a rapid glucose release mechanism during energy demand.
Glycogenolysis Explained: Mechanisms and Regulation
Glycogenolysis is the biochemical pathway responsible for breaking down glycogen into glucose-1-phosphate, which is then converted to glucose-6-phosphate to provide energy during fasting or intense exercise. This process is primarily regulated by hormonal signals such as glucagon and epinephrine, which activate glycogen phosphorylase through phosphorylation cascades involving protein kinase A and phosphorylase kinase. Key enzymes include glycogen phosphorylase, which cleaves a-1,4-glycosidic bonds, and debranching enzyme that remodels the glycogen molecule, ensuring efficient glucose mobilization for maintaining blood glucose homeostasis.
Metabolic Context: When Does Each Process Occur?
Gluconeogenesis primarily occurs during prolonged fasting, intense exercise, or starvation, enabling the liver and kidneys to produce glucose from non-carbohydrate precursors like lactate, glycerol, and amino acids to maintain blood glucose levels. Glycogenolysis happens when the body experiences sudden energy demands such as between meals or during short-term fasting, leading to the breakdown of stored glycogen in the liver and muscles to release glucose or glucose-6-phosphate for immediate energy use. Both processes are tightly regulated by hormones like glucagon and insulin to balance blood glucose homeostasis under varying metabolic conditions.
Substrate Sources for Gluconeogenesis vs Glycogenolysis
Gluconeogenesis primarily uses non-carbohydrate substrates such as lactate, glycerol, and glucogenic amino acids to synthesize glucose, especially during prolonged fasting or intense exercise. Glycogenolysis involves the breakdown of stored glycogen into glucose-1-phosphate, which is converted to glucose-6-phosphate for immediate energy needs in liver and muscle tissues. These processes ensure glucose availability, with gluconeogenesis sustaining blood glucose when glycogen stores are depleted.
Hormonal Regulation: Insulin, Glucagon, and Stress Hormones
Hormonal regulation of gluconeogenesis and glycogenolysis involves insulin, glucagon, and stress hormones such as cortisol and epinephrine. Insulin suppresses both gluconeogenesis and glycogenolysis to lower blood glucose levels, while glucagon stimulates these pathways to increase glucose production during fasting. Stress hormones enhance glycogenolysis and gluconeogenesis to provide rapid energy availability during physiological stress.
Cellular Localization: Where Do Processes Happen?
Gluconeogenesis primarily occurs in the cytoplasm of liver and kidney cells, with key steps also taking place in the mitochondria, particularly the conversion of pyruvate to oxaloacetate. Glycogenolysis takes place mainly in the cytoplasm of liver and muscle cells, where glycogen is broken down into glucose-1-phosphate to meet energy demands. Both pathways are tightly regulated and compartmentalized to coordinate glucose homeostasis effectively.
Physiological Significance and Energy Production
Gluconeogenesis maintains blood glucose levels during prolonged fasting by synthesizing glucose from non-carbohydrate sources like lactate and amino acids, ensuring continuous energy supply to tissues, especially the brain. Glycogenolysis rapidly mobilizes stored glycogen in liver and muscle cells to produce glucose-6-phosphate, providing immediate energy during short-term fasting or intense physical activity. Both pathways are critical for energy homeostasis, with gluconeogenesis supporting long-term energy demands and glycogenolysis delivering quick energy bursts.
Clinical Relevance: Disorders and Therapeutic Implications
Gluconeogenesis and glycogenolysis play critical roles in maintaining blood glucose levels, with dysregulation leading to disorders such as glycogen storage diseases and type 2 diabetes. Deficiencies in enzymes like glucose-6-phosphatase in glycogenolysis cause hypoglycemia and hepatomegaly, while impaired gluconeogenesis contributes to lactic acidosis and ketoacidosis in metabolic conditions. Therapeutic strategies targeting these pathways include enzyme replacement, dietary management, and glucose-lowering agents that modulate hepatic glucose output to restore metabolic balance.
Key Differences and Comparative Summary
Gluconeogenesis is the metabolic process that synthesizes glucose from non-carbohydrate substrates like lactate, glycerol, and amino acids, primarily occurring in the liver and kidneys during fasting or intense exercise. In contrast, glycogenolysis involves the breakdown of glycogen stores into glucose-1-phosphate to quickly increase blood glucose levels during short-term energy demands. While gluconeogenesis is an energy-consuming anabolic pathway taking hours to activate, glycogenolysis is a rapid catabolic process providing immediate glucose, highlighting their complementary roles in maintaining blood glucose homeostasis.
Gluconeogenesis Infographic
 libterm.com
libterm.com