Enantiomers are molecules that are mirror images of each other but cannot be superimposed, playing a crucial role in chemistry and pharmaceuticals due to their different biological activities. Understanding how enantiomers interact with biological systems helps in designing drugs with enhanced efficacy and reduced side effects. Discover more about the significance of enantiomers and how they impact your health by reading the full article.
Table of Comparison
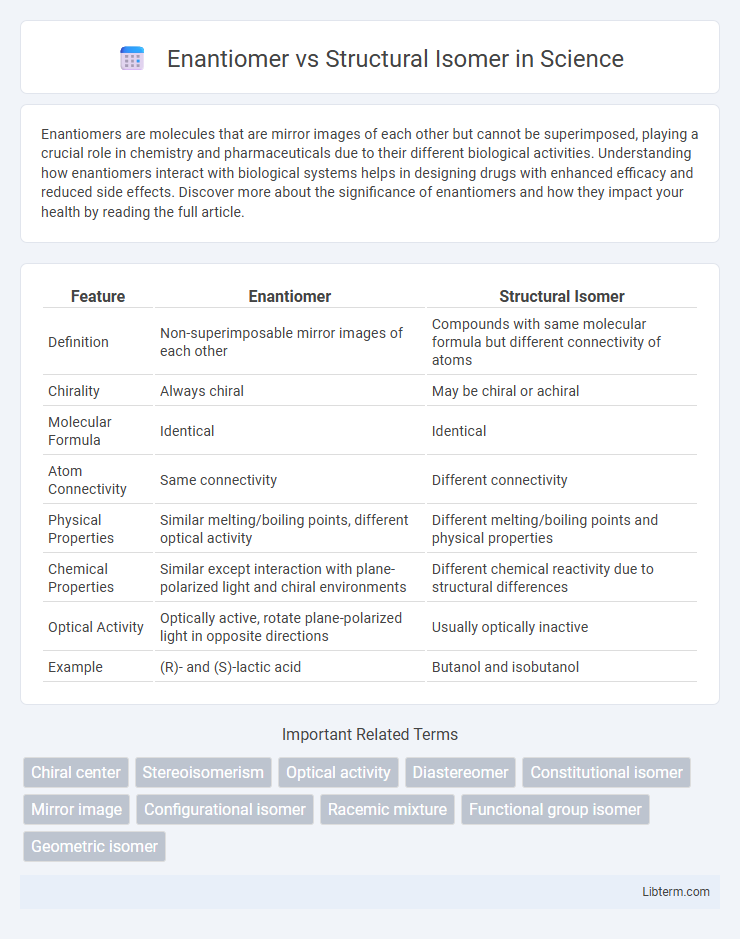
| Feature | Enantiomer | Structural Isomer |
|---|---|---|
| Definition | Non-superimposable mirror images of each other | Compounds with same molecular formula but different connectivity of atoms |
| Chirality | Always chiral | May be chiral or achiral |
| Molecular Formula | Identical | Identical |
| Atom Connectivity | Same connectivity | Different connectivity |
| Physical Properties | Similar melting/boiling points, different optical activity | Different melting/boiling points and physical properties |
| Chemical Properties | Similar except interaction with plane-polarized light and chiral environments | Different chemical reactivity due to structural differences |
| Optical Activity | Optically active, rotate plane-polarized light in opposite directions | Usually optically inactive |
| Example | (R)- and (S)-lactic acid | Butanol and isobutanol |
Introduction to Isomerism in Chemistry
Isomerism in chemistry refers to compounds with the same molecular formula but different structural arrangements, leading to distinct properties. Enantiomers are stereoisomers that are non-superimposable mirror images of each other, exhibiting chirality and optical activity. Structural isomers differ in the connectivity of atoms within the molecule, resulting in variations such as chain isomers, position isomers, and functional group isomers.
Definition of Enantiomers
Enantiomers are pairs of stereoisomers that are non-superimposable mirror images of each other, differing in the spatial arrangement of atoms around a chiral center. These molecules share the same molecular formula and connectivity but have opposite configurations, leading to distinct optical activities, such as rotating plane-polarized light in opposite directions. Structural isomers, in contrast, differ in the actual connectivity of atoms, resulting in different chemical properties and molecular structures rather than mirror-image relationships.
Definition of Structural Isomers
Structural isomers are compounds that share the same molecular formula but differ in the connectivity of their atoms, leading to distinct chemical and physical properties. Unlike enantiomers, which are non-superimposable mirror images with identical connectivity, structural isomers may vary in the arrangement of atoms, such as chain branching or functional group position. Key examples include chain isomers, positional isomers, and functional isomers, all classified under structural isomerism due to their unique atomic linkages.
Key Differences Between Enantiomers and Structural Isomers
Enantiomers are stereoisomers that are non-superimposable mirror images of each other, differing primarily in spatial arrangement, whereas structural isomers vary in the connectivity of their atoms, resulting in different molecular formulas or structures. Enantiomers share the same molecular formula and sequence of bonded atoms but differ in the 3D orientation around a chiral center, which affects their optical activity and biological interactions, while structural isomers include different types such as chain, positional, or functional group isomers, leading to distinct chemical properties. The key difference lies in enantiomers' identical connectivity with divergent stereochemistry versus structural isomers' distinct atomic connectivity and structure.
Chemical Properties: Enantiomers vs Structural Isomers
Enantiomers share identical chemical properties in achiral environments but differ significantly in their interaction with plane-polarized light and chiral reagents, exhibiting optical activity and stereospecific reactions. Structural isomers display distinct chemical properties due to differences in the connectivity of atoms, leading to variations in reactivity, boiling points, and functional group behavior. The stereochemical arrangement in enantiomers mainly influences reactions in chiral contexts, while structural isomers' chemical diversity affects broader chemical behavior.
Physical Properties Comparison
Enantiomers possess identical physical properties such as melting point, boiling point, and solubility, except they differ in the direction they rotate plane-polarized light (optical activity). Structural isomers exhibit varied physical properties including distinct melting points, boiling points, densities, and refractive indices due to differences in atom connectivity. These disparity patterns make enantiomers indistinguishable by most physical methods except chiroptical techniques, whereas structural isomers can often be differentiated by standard physical measurements.
Importance in Pharmaceuticals
Enantiomers and structural isomers are crucial in pharmaceuticals due to their distinct effects on drug activity and safety. Enantiomers, being mirror-image molecules, often exhibit different biological activities or therapeutic effects, with one enantiomer potentially providing the desired effect while the other causes adverse reactions. Structural isomers, varying in connectivity of atoms, can lead to significant differences in pharmacodynamics and pharmacokinetics, influencing drug efficacy and metabolism.
Methods of Identification and Analysis
Enantiomers are identified using polarimetry, which measures the rotation of plane-polarized light, and chiral chromatography methods such as HPLC with chiral stationary phases. Structural isomers are primarily analyzed through spectroscopic techniques like NMR (nuclear magnetic resonance) and mass spectrometry, which differentiate based on connectivity and functional groups. Advanced methods like IR spectroscopy and 2D NMR further assist in distinguishing subtle differences in bonding and molecular frameworks.
Real-World Examples
Enantiomers, such as the two forms of the drug thalidomide, exhibit identical physical properties but differ in their biological effects, with one causing therapeutic benefits and the other harmful side effects. Structural isomers, exemplified by butanol and diethyl ether, have the same molecular formula but different connectivity, resulting in distinct chemical reactivity and uses, like solvents or fuels. The real-world impact of these isomer types is critical in pharmaceuticals and industrial applications, where molecular arrangement dictates function and safety.
Conclusion: Choosing the Right Isomer for Applications
Enantiomers exhibit identical physical properties but differ in optical activity, making them crucial in pharmaceuticals where stereochemistry affects drug efficacy and safety. Structural isomers vary in connectivity and physical properties, offering diverse chemical functionalities beneficial in materials science and synthetic chemistry. Selecting the appropriate isomer depends on the desired application, with enantiomers preferred for chiral-specific interactions and structural isomers chosen for varied chemical behavior and reactivity.
Enantiomer Infographic
 libterm.com
libterm.com