Enantiomeric compounds possess molecules that are non-superimposable mirror images, crucial in fields like pharmaceuticals for their differing biological activities. Understanding enantiomeric purity impacts drug efficacy and safety, making it essential in chemical research and product development. Explore the article to discover how enantiomeric properties influence your work and health.
Table of Comparison
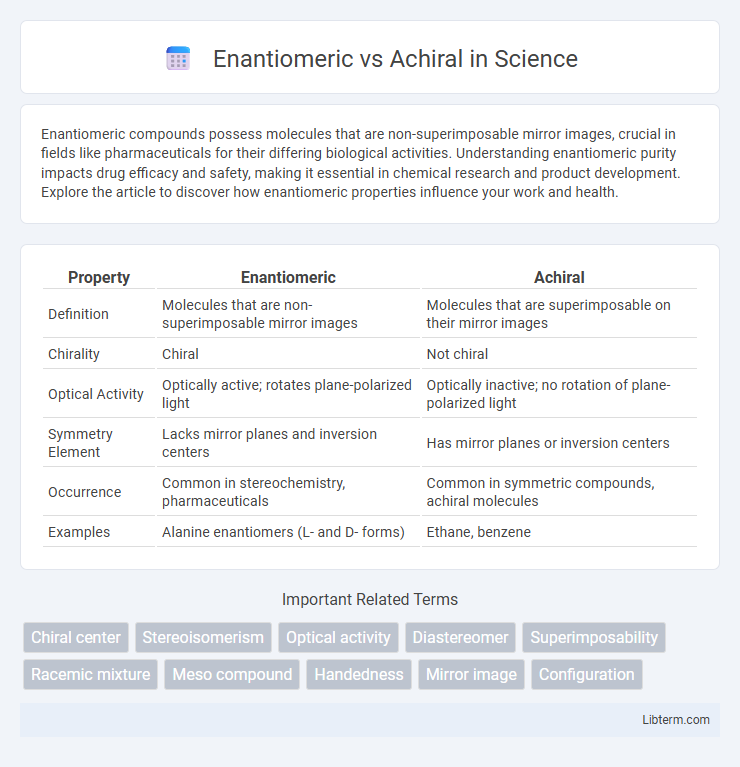
| Property | Enantiomeric | Achiral |
|---|---|---|
| Definition | Molecules that are non-superimposable mirror images | Molecules that are superimposable on their mirror images |
| Chirality | Chiral | Not chiral |
| Optical Activity | Optically active; rotates plane-polarized light | Optically inactive; no rotation of plane-polarized light |
| Symmetry Element | Lacks mirror planes and inversion centers | Has mirror planes or inversion centers |
| Occurrence | Common in stereochemistry, pharmaceuticals | Common in symmetric compounds, achiral molecules |
| Examples | Alanine enantiomers (L- and D- forms) | Ethane, benzene |
Introduction to Chirality in Chemistry
Chirality in chemistry refers to the geometric property where a molecule cannot be superimposed on its mirror image, leading to the existence of enantiomers--pairs of chiral molecules that are non-superimposable mirror images. Enantiomers have identical physical properties except for the direction in which they rotate plane-polarized light and their interactions with other chiral substances. Achiral molecules lack this property and are superimposable on their mirror images, resulting in no optical activity.
Defining Enantiomers: Key Characteristics
Enantiomers are chiral molecules that exist as non-superimposable mirror images, each having opposite configurations at one or more stereogenic centers. Key characteristics include identical physical properties except for the direction in which they rotate plane-polarized light and their distinct interactions with other chiral compounds. Achiral molecules lack stereogenic centers and are superimposable on their mirror images, exhibiting no optical activity.
Understanding Achiral Molecules
Achiral molecules lack chiral centers and are superimposable on their mirror images, meaning they do not exhibit optical activity. These molecules have symmetrical structures that prevent the formation of non-superimposable mirror images, distinguishing them from enantiomers. Understanding achiral molecules is essential in stereochemistry for predicting molecular behavior and interactions in chemical reactions.
Structural Differences: Enantiomeric vs Achiral Compounds
Enantiomeric compounds possess chiral centers, usually carbon atoms bonded to four distinct substituents, creating non-superimposable mirror images known as enantiomers. Achiral compounds lack such asymmetry and either have symmetrical substituents or internal planes of symmetry that prevent chirality. The structural difference lies in the spatial arrangement of atoms, where enantiomers exhibit stereoisomerism, while achiral molecules are superimposable on their mirror images.
Optical Activity: Enantiomers vs Achiral Substances
Enantiomers exhibit optical activity by rotating plane-polarized light either clockwise (dextrorotatory) or counterclockwise (levorotatory), a property critical in stereochemistry and chiral drug design. Achiral substances, lacking asymmetry, do not rotate plane-polarized light and are therefore optically inactive. The measurement of optical rotation using polarimetry distinguishes enantiomers from achiral compounds in analytical chemistry and pharmaceutical applications.
Methods for Identifying Enantiomers and Achiral Compounds
Methods for identifying enantiomers primarily involve chiral chromatography and polarimetry, which measure optical rotation to distinguish between left- and right-handed isomers. Nuclear Magnetic Resonance (NMR) spectroscopy using chiral shift reagents also helps differentiate enantiomers by producing distinct spectral signals. Achiral compounds, lacking optical activity, are typically identified by standard chromatographic techniques and achiral NMR spectra without enantiomer-specific shifts.
Significance in Pharmaceuticals: Enantiomeric vs Achiral Drugs
Enantiomeric drugs exhibit distinct biological activities due to their chiral nature, influencing drug efficacy and safety profiles in pharmaceuticals. Achiral drugs lack stereoisomers, often resulting in uniform pharmacological effects but potentially limiting therapeutic specificity. Understanding the significance of enantiomeric versus achiral drugs is crucial for optimizing drug design and personalized medicine outcomes.
Synthesis and Separation Techniques
Enantiomeric compounds exhibit chirality, requiring advanced synthesis techniques such as asymmetric catalysis and chiral auxiliaries to produce specific enantiomers with high optical purity. Separation techniques for enantiomers commonly involve chiral chromatography, including HPLC with chiral stationary phases or supercritical fluid chromatography, which exploit differential interactions with chiral selectors. Achiral compounds, lacking stereoisomers, typically undergo standard synthetic methods without stereochemical control and use conventional separation methods like recrystallization or achiral column chromatography.
Applications in Chemical and Biological Systems
Enantiomeric compounds play a crucial role in pharmaceuticals by exhibiting distinct biological activities and interactions with chiral receptors, impacting drug efficacy and safety. Achiral molecules, lacking handedness, are essential in industrial catalysis and material science where stereospecificity is non-critical, ensuring consistent product outcomes. In biochemical systems, enantiomers influence enzyme-substrate binding and metabolic pathways, making chirality vital for drug design and molecular recognition processes.
Conclusion: Implications of Chirality in Science
Chirality profoundly influences molecular interactions, as enantiomeric compounds exhibit distinct biological activities due to their non-superimposable mirror images, whereas achiral molecules lack this stereochemical complexity. This distinction is crucial in pharmaceuticals, where enantiomeric purity can determine a drug's efficacy and safety. Understanding chirality enables targeted drug design, improved material properties, and advances in stereoselective synthesis, highlighting its central role in chemistry and biology.
Enantiomeric Infographic
 libterm.com
libterm.com