Photophilic organisms thrive in environments with abundant light, adapting their biological processes to maximize energy absorption from sunlight. These organisms often display vibrant pigmentation and sophisticated mechanisms for photosynthesis or light sensitivity. Explore the rest of the article to understand how your environment influences photophilic life and their ecological significance.
Table of Comparison
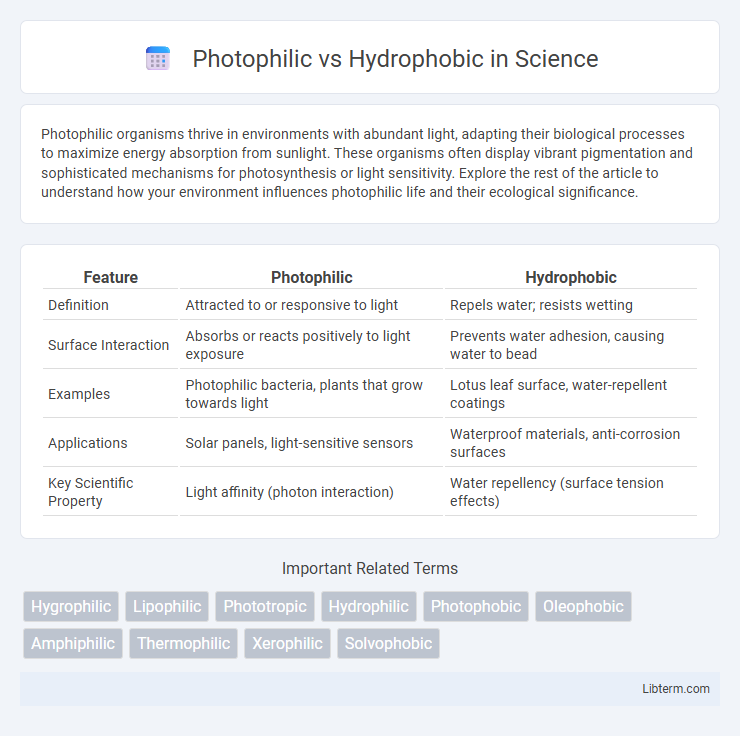
| Feature | Photophilic | Hydrophobic |
|---|---|---|
| Definition | Attracted to or responsive to light | Repels water; resists wetting |
| Surface Interaction | Absorbs or reacts positively to light exposure | Prevents water adhesion, causing water to bead |
| Examples | Photophilic bacteria, plants that grow towards light | Lotus leaf surface, water-repellent coatings |
| Applications | Solar panels, light-sensitive sensors | Waterproof materials, anti-corrosion surfaces |
| Key Scientific Property | Light affinity (photon interaction) | Water repellency (surface tension effects) |
Understanding Photophilic and Hydrophobic Terminology
Photophilic materials exhibit an affinity for light, often absorbing or interacting with photons to facilitate processes like photosynthesis or photochemical reactions, while hydrophobic substances repel water, preventing absorption and interaction with moisture. Understanding photophilic and hydrophobic terminology involves recognizing their fundamental chemical properties: photophilic relates to light-attracting molecules or surfaces, whereas hydrophobic describes water-repelling characteristics commonly found in nonpolar substances such as oils and waxes. These distinctions are critical in fields like material science, biology, and chemistry for designing light-responsive materials and water-resistant coatings.
Fundamental Differences: Photophilic vs. Hydrophobic
Photophilic materials exhibit a strong affinity for light, often enhancing or absorbing specific wavelengths, while hydrophobic materials repel water, minimizing surface wetting and adhesion. Photophilic surfaces are engineered to maximize light interaction for applications like photovoltaics or sensors, whereas hydrophobic surfaces prioritize water resistance and self-cleaning properties. The fundamental difference lies in their interaction with physical stimuli: light for photophilic materials versus water for hydrophobic materials.
Chemical Properties and Molecular Structure
Photophilic materials exhibit strong affinity for light and often contain conjugated systems or aromatic rings that facilitate photon absorption, enhancing photoreactivity. Hydrophobic molecules possess nonpolar, hydrocarbon-rich structures that repel water by minimizing polar interactions, typically characterized by alkyl chains or fluorinated groups. Differences in chemical properties arise from molecular polarity, where photophilic compounds tend to have delocalized electrons for light interaction, while hydrophobic substances exhibit high water contact angles due to their nonpolar surfaces.
Occurrence in Nature and Everyday Life
Photophilic organisms and materials actively absorb and respond to light, commonly seen in plants and photosynthetic algae that rely on sunlight for energy conversion, while hydrophobic substances repel water, evident in natural examples like lotus leaves and insect exoskeletons that prevent water adhesion. In everyday life, photophilic properties are utilized in solar panels and photography, whereas hydrophobic coatings are widely applied to textiles, electronics, and automotive surfaces to enhance water resistance. These opposing characteristics influence ecological interactions and technological developments through light attraction and water repellence mechanisms.
Photophilic Materials: Characteristics and Examples
Photophilic materials exhibit a strong affinity for light, often undergoing physical or chemical changes when exposed to specific wavelengths, making them ideal for applications in sensors, solar cells, and photodynamic therapy. These materials typically possess photoactive molecules or functional groups such as azobenzene, spiropyran, or titanium dioxide nanoparticles that respond to ultraviolet or visible light stimuli. Examples of photophilic materials include photochromic polymers, perovskite solar absorbers, and titanium dioxide-based photocatalysts, which enhance light absorption and energy conversion efficiency.
Hydrophobic Materials: Characteristics and Examples
Hydrophobic materials repel water due to their low surface energy and non-polar molecular structure, causing water droplets to bead up rather than spread. Common hydrophobic examples include polytetrafluoroethylene (PTFE), silicone, and wax coatings, which are widely used in waterproof textiles, non-stick cookware, and protective coatings. These materials demonstrate high water resistance, chemical stability, and durability, making them essential for applications requiring moisture protection and corrosion resistance.
Roles in Biological Systems and Environmental Impact
Photophilic molecules absorb or utilize light energy, playing crucial roles in photosynthesis and vision by converting light into chemical or neural signals. Hydrophobic substances repel water, essential for forming cellular membranes and facilitating lipid-based energy storage, impacting biological compartmentalization and signaling. Environmentally, photophilic compounds drive ecosystems by supporting primary production, while hydrophobic pollutants accumulate in water bodies, affecting aquatic life and bioaccumulation.
Industrial and Technological Applications
Photophilic materials exhibit strong affinity to light, making them ideal for advanced optical sensors and photovoltaic cells in industrial applications. Hydrophobic surfaces repel water, enhancing durability and efficiency in technology sectors such as corrosion-resistant coatings and microfluidic devices. The integration of photophilic and hydrophobic properties enables the development of smart materials for self-cleaning solar panels and water-repellent light-activated sensors.
Recent Advances in Material Science
Recent advances in material science have led to the development of photophilic and hydrophobic surfaces with enhanced functionalities for applications in self-cleaning, anti-fogging, and oil-water separation technologies. Nanostructured coatings and stimuli-responsive polymers exhibit controlled wettability by switching between photophilic (water-attracting) and hydrophobic (water-repelling) states under light irradiation, enabling smart surface modulation. Cutting-edge research integrates photocatalytic nanoparticles and tunable surface energy materials to optimize performance, durability, and environmental responsiveness of these innovative surfaces.
Future Trends and Innovative Uses
Photophilic materials, which attract light, are increasingly integrated into advanced solar energy systems and smart windows to enhance energy efficiency and indoor comfort. Hydrophobic surfaces are evolving through nanotechnology to create self-cleaning, anti-corrosion coatings for electronics and healthcare devices, improving durability and hygiene. Future trends focus on hybrid materials combining photophilic and hydrophobic properties for innovative applications in wearable technology and environmental sensors.
Photophilic Infographic
 libterm.com
libterm.com