Chromatography is a powerful analytical technique used to separate and analyze the components of complex mixtures based on their physical or chemical properties. It plays a crucial role in fields such as pharmaceuticals, environmental science, and food safety by allowing precise identification and quantification of substances. Explore the rest of this article to uncover how chromatography can enhance your scientific analyses.
Table of Comparison
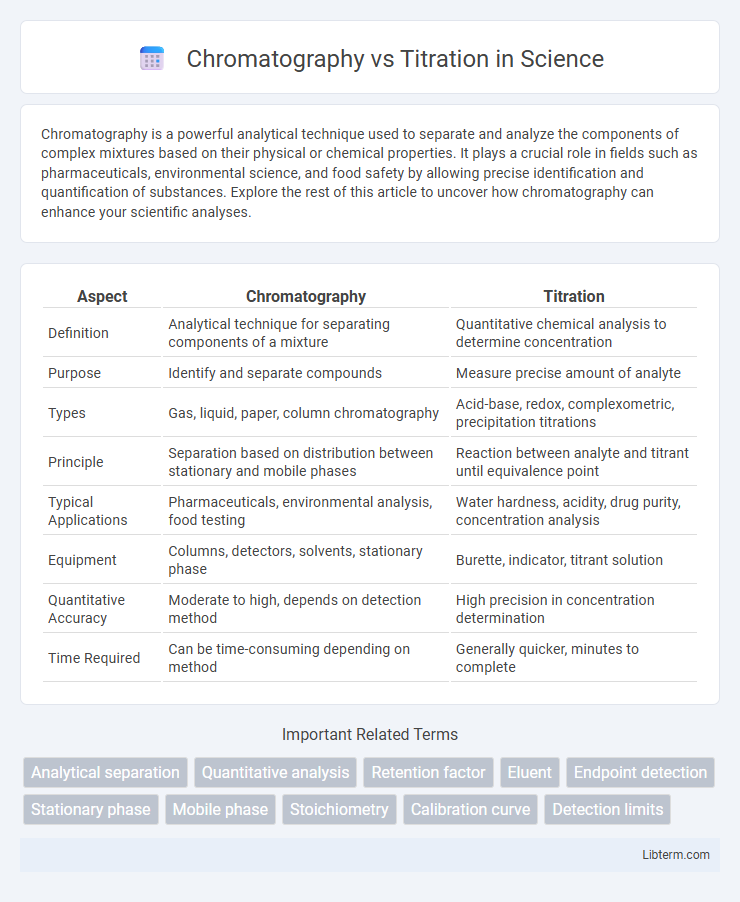
| Aspect | Chromatography | Titration |
|---|---|---|
| Definition | Analytical technique for separating components of a mixture | Quantitative chemical analysis to determine concentration |
| Purpose | Identify and separate compounds | Measure precise amount of analyte |
| Types | Gas, liquid, paper, column chromatography | Acid-base, redox, complexometric, precipitation titrations |
| Principle | Separation based on distribution between stationary and mobile phases | Reaction between analyte and titrant until equivalence point |
| Typical Applications | Pharmaceuticals, environmental analysis, food testing | Water hardness, acidity, drug purity, concentration analysis |
| Equipment | Columns, detectors, solvents, stationary phase | Burette, indicator, titrant solution |
| Quantitative Accuracy | Moderate to high, depends on detection method | High precision in concentration determination |
| Time Required | Can be time-consuming depending on method | Generally quicker, minutes to complete |
Introduction to Chromatography and Titration
Chromatography is an analytical technique used to separate and identify components within a mixture based on their distribution between a stationary phase and a mobile phase. Titration is a quantitative chemical analysis method that determines the concentration of an unknown solution by reacting it with a standard solution of known concentration. Both techniques play crucial roles in chemical analysis, with chromatography excelling in mixture separation and titration providing precise concentration measurements.
Principles Underlying Chromatography
Chromatography operates on the principle of separating components based on their differential affinities between a stationary phase and a mobile phase, allowing substances to be isolated and identified effectively. This technique relies on the distribution equilibrium of analytes as they traverse the chromatographic medium, with separation achieved through adsorption, partition, ion exchange, or size exclusion mechanisms. Unlike titration, which depends on stoichiometric reactions to determine concentration, chromatography provides qualitative and quantitative analysis by exploiting molecular interactions within various chromatographic systems such as gas chromatography, liquid chromatography, or thin-layer chromatography.
Fundamentals of Titration Techniques
Titration techniques rely on precise measurement of the volume of a titrant added to a solution to determine analyte concentration via stoichiometric reaction endpoints. Indicators or potentiometric methods detect the endpoint, ensuring high accuracy in acid-base, redox, complexometric, or precipitation titrations. Chromatography separates components based on differential affinities, but titration remains fundamental for quantitative chemical analysis through direct volumetric methods.
Key Differences Between Chromatography and Titration
Chromatography separates mixtures into individual components based on differences in their distribution between a stationary phase and a mobile phase, making it ideal for qualitative and quantitative analysis of complex samples. Titration measures the concentration of a specific analyte by reacting it with a standard solution, providing precise quantitative results through volume measurement during the endpoint. While chromatography offers separation and identification of multiple substances simultaneously, titration is limited to determining the concentration of a single component through chemical reaction.
Types of Chromatography Methods
Chromatography encompasses several types, including gas chromatography (GC) for volatile compounds, liquid chromatography (LC) for a broad range of substances, and thin-layer chromatography (TLC) commonly used for qualitative analysis. High-performance liquid chromatography (HPLC) offers high resolution and sensitivity, making it suitable for complex mixtures, while ion-exchange chromatography separates ions based on charge differences. Unlike titration, which measures analyte concentration through chemical reactions, chromatography provides detailed separation and identification of components within a mixture.
Various Titration Procedures
Various titration procedures include acid-base, redox, complexometric, and precipitation titrations, each tailored to specific chemical reactions and analytes. Acid-base titrations utilize indicators or pH meters to determine the equivalence point, while redox titrations rely on oxidation-reduction reactions with appropriate titrants such as potassium permanganate or iodine. Complexometric titrations often employ EDTA to quantify metal ions, and precipitation titrations use the formation of insoluble salts to analyze concentrations, demonstrating the versatility and precision of titration techniques compared to chromatographic separation methods.
Common Applications in Laboratory Analysis
Chromatography is widely used for separating complex mixtures and identifying components in pharmaceuticals, environmental samples, and food products, offering precise qualitative and quantitative analysis. Titration excels in determining the concentration of specific substances, particularly in acid-base analysis, water quality testing, and pharmaceutical formulations. Both techniques are essential in laboratory analysis for ensuring accuracy in chemical composition and concentration measurements across diverse scientific fields.
Sensitivity and Accuracy Comparison
Chromatography offers higher sensitivity compared to titration, detecting trace levels of analytes with precision. Accuracy in chromatography is enhanced by its ability to separate complex mixtures, minimizing interference effects that commonly affect titration results. Titration provides reliable accuracy for concentrated solutions but lacks the sensitivity needed for low-level analyte detection.
Advantages and Limitations of Each Technique
Chromatography offers high sensitivity and the ability to separate complex mixtures, making it ideal for qualitative and quantitative analysis of multi-component samples; however, it requires expensive equipment and skilled operators, and can be time-consuming. Titration is a cost-effective, straightforward method providing precise concentration measurements, especially for acid-base reactions, but it lacks the capacity to analyze mixtures with multiple components and is less suitable for trace-level detection. Both techniques complement each other: chromatography excels in detailed component separation and identification, while titration is preferred for rapid, routine quantitative analysis.
Choosing the Right Analytical Method
Choosing the right analytical method between chromatography and titration depends on the specific requirements of accuracy, sensitivity, and sample complexity. Chromatography excels in separating and identifying compounds within complex mixtures, offering high precision and qualitative data. Titration suits quantitative analysis of known substances with simpler sample matrices, providing faster results and cost-effective measurements.
Chromatography Infographic
 libterm.com
libterm.com