Case-control studies compare individuals with a specific condition to those without to identify factors that may contribute to the disease. This research design is particularly effective for studying rare diseases and assessing multiple risk factors simultaneously. Explore the article to learn how case-control studies can advance your understanding of disease causation.
Table of Comparison
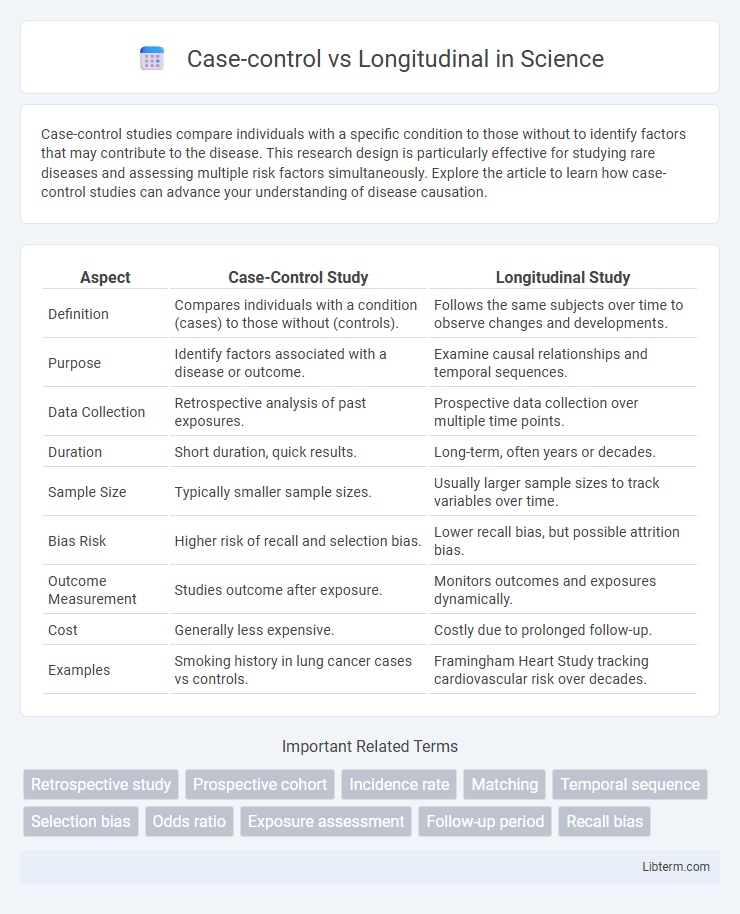
| Aspect | Case-Control Study | Longitudinal Study |
|---|---|---|
| Definition | Compares individuals with a condition (cases) to those without (controls). | Follows the same subjects over time to observe changes and developments. |
| Purpose | Identify factors associated with a disease or outcome. | Examine causal relationships and temporal sequences. |
| Data Collection | Retrospective analysis of past exposures. | Prospective data collection over multiple time points. |
| Duration | Short duration, quick results. | Long-term, often years or decades. |
| Sample Size | Typically smaller sample sizes. | Usually larger sample sizes to track variables over time. |
| Bias Risk | Higher risk of recall and selection bias. | Lower recall bias, but possible attrition bias. |
| Outcome Measurement | Studies outcome after exposure. | Monitors outcomes and exposures dynamically. |
| Cost | Generally less expensive. | Costly due to prolonged follow-up. |
| Examples | Smoking history in lung cancer cases vs controls. | Framingham Heart Study tracking cardiovascular risk over decades. |
Introduction to Case-Control and Longitudinal Study Designs
Case-control studies compare individuals with a specific outcome (cases) to those without (controls) to identify potential risk factors by retrospectively examining exposures. Longitudinal studies track the same subjects over time to observe how exposures influence the development of outcomes, allowing for the assessment of temporal relationships and changes. Both designs are fundamental in epidemiology, with case-control studies being efficient for rare diseases and longitudinal studies providing stronger evidence for causality.
Defining Case-Control Studies
Case-control studies are observational research designs that compare individuals with a specific condition or disease (cases) to those without the condition (controls) to identify factors associated with the outcome. These studies are retrospective, focusing on past exposures or risk factors by analyzing existing records or participant recall. Case-control studies are efficient for investigating rare diseases or conditions with long latency periods and provide relative risk estimates through odds ratios.
Defining Longitudinal Studies
Longitudinal studies track the same subjects over an extended period, allowing researchers to observe changes and development within individuals. These studies provide valuable data on temporal sequences and causal relationships by repeatedly measuring variables at multiple time points. Unlike case-control studies, longitudinal designs capture dynamic processes and trends rather than relying on retrospective comparisons.
Key Differences Between Case-Control and Longitudinal Studies
Case-control studies compare individuals with a specific outcome (cases) to those without (controls) to identify factors associated with the outcome by looking retrospectively. Longitudinal studies track the same subjects over a period of time to observe how exposures influence the development of outcomes prospectively. Key differences include temporal directionality--retrospective in case-control and prospective in longitudinal designs--and the ability of longitudinal studies to establish temporal sequences and infer causality more robustly.
Advantages of Case-Control Studies
Case-control studies offer significant efficiency in investigating rare diseases or outcomes by comparing subjects with the condition (cases) to those without (controls), often requiring fewer resources and time than longitudinal designs. They enable the assessment of multiple exposures related to a single outcome and are particularly advantageous for generating hypotheses about risk factors. This design also facilitates quicker data collection, making it highly suitable for preliminary research in epidemiology and public health.
Advantages of Longitudinal Studies
Longitudinal studies offer significant advantages by tracking the same subjects over extended periods, allowing researchers to observe changes and establish temporal sequences between exposure and outcome. This design reduces recall bias and provides stronger evidence for causal relationships compared to case-control studies, which rely on retrospective data. The ability to monitor developmental patterns and risk factors in real time enhances the accuracy and depth of epidemiological and behavioral research findings.
Limitations of Case-Control Studies
Case-control studies are prone to recall bias since participants may not accurately remember past exposures, which can affect the validity of findings. They cannot establish temporal relationships, limiting the ability to infer causality between risk factors and outcomes. Selection bias is another issue, as control groups may not be representative of the general population, reducing the study's generalizability.
Limitations of Longitudinal Studies
Longitudinal studies often face significant limitations such as high costs and extended timeframes, which can hinder timely results and feasibility. Participant attrition over the study period may introduce bias and reduce the reliability of findings. Additionally, maintaining consistent measurement methods and controlling for confounding variables over time presents challenges that can impact data validity.
Choosing the Right Study Design for Research Objectives
Case-control studies efficiently identify risk factors by comparing subjects with a condition to those without, ideal for rare diseases or outcomes. Longitudinal studies track the same subjects over time, providing insights into temporal relationships and causal inferences for chronic conditions. Selecting the right design depends on research objectives, disease frequency, and resource availability, ensuring validity and reliability in findings.
Case-Control vs Longitudinal: Summary and Final Considerations
Case-control studies compare individuals with a specific outcome to those without, efficiently identifying potential risk factors but often limited by retrospective bias. Longitudinal studies track subjects over time, providing stronger evidence for causal relationships through temporal data but requiring more resources and extended follow-up. Choosing between case-control and longitudinal designs depends on research goals, resource availability, and the need for causal inference versus exploratory analysis.
Case-control Infographic
 libterm.com
libterm.com