Atomic-level understanding of materials enables breakthroughs in technology, energy storage, and quantum computing. Precise control over atomic interactions can revolutionize industries and improve your daily life through enhanced performance and efficiency. Explore the rest of this article to discover how atomic science shapes the future.
Table of Comparison
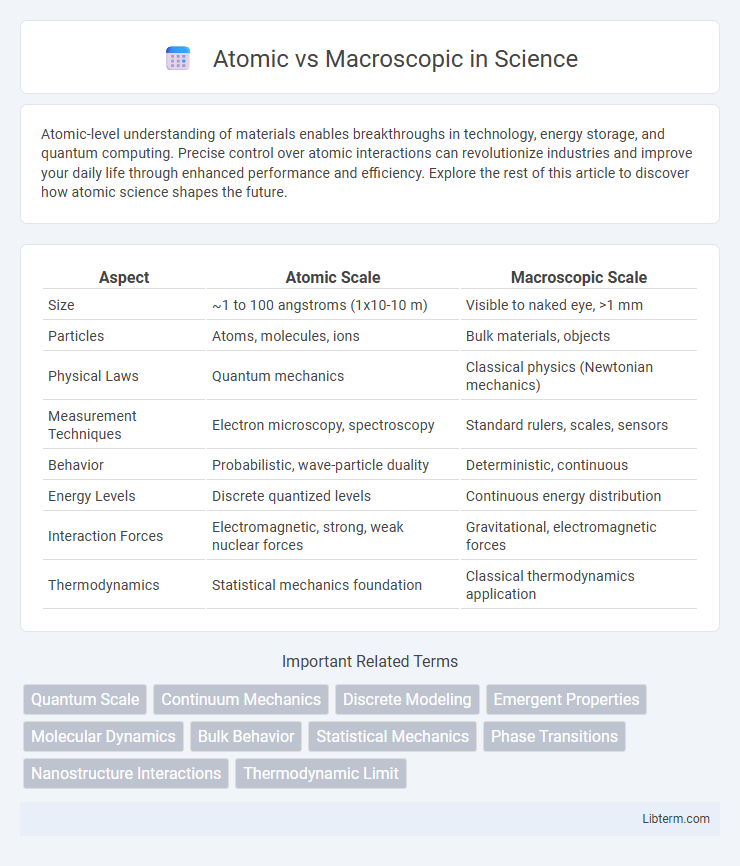
| Aspect | Atomic Scale | Macroscopic Scale |
|---|---|---|
| Size | ~1 to 100 angstroms (1x10-10 m) | Visible to naked eye, >1 mm |
| Particles | Atoms, molecules, ions | Bulk materials, objects |
| Physical Laws | Quantum mechanics | Classical physics (Newtonian mechanics) |
| Measurement Techniques | Electron microscopy, spectroscopy | Standard rulers, scales, sensors |
| Behavior | Probabilistic, wave-particle duality | Deterministic, continuous |
| Energy Levels | Discrete quantized levels | Continuous energy distribution |
| Interaction Forces | Electromagnetic, strong, weak nuclear forces | Gravitational, electromagnetic forces |
| Thermodynamics | Statistical mechanics foundation | Classical thermodynamics application |
Introduction to Atomic and Macroscopic Scales
Atomic scales refer to dimensions on the order of angstroms (10^-10 meters), where individual atoms and molecules govern physical properties and behaviors. Macroscopic scales span from micrometers to meters, encompassing objects visible to the naked eye and governed by classical physics principles. Understanding the distinction between atomic and macroscopic scales is crucial for fields such as materials science, nanotechnology, and quantum mechanics.
Defining Atomic Scale: Basic Concepts
The atomic scale refers to dimensions on the order of angstroms (10^-10 meters), where individual atoms and their interactions dominate physical properties. At this scale, quantum mechanics governs particle behavior, distinguishing it from macroscopic phenomena observable in everyday objects measured in millimeters to meters. Understanding electron orbitals, atomic nuclei, and intermolecular forces is fundamental to defining properties like conductivity, magnetism, and chemical reactivity at the atomic level.
Understanding the Macroscopic World
Understanding the macroscopic world involves analyzing aggregates of atoms and molecules, where classical physics principles govern observable phenomena such as motion, temperature, and pressure. Unlike atomic-scale interactions described by quantum mechanics, macroscopic systems exhibit predictable behavior that can be measured directly through instruments and sensory perception. Studying macroscopic properties enables advancements in engineering, material science, and thermodynamics by linking microscopic atomic structures to large-scale physical applications.
Fundamental Differences Between Atomic and Macroscopic Levels
Atomic level phenomena involve interactions governed by quantum mechanics, where particles such as electrons, protons, and neutrons exhibit wave-particle duality and discrete energy states. Macroscopic level behavior follows classical physics principles, characterized by continuous matter and energy distributions observable in everyday objects. The fundamental difference lies in scale and governing laws: atomic systems require quantum descriptions for accurate predictions, whereas macroscopic systems are effectively described by Newtonian mechanics and thermodynamics.
Observable Properties: Atomic vs. Macroscopic
Observable properties at the atomic level include discrete energy states, electron configurations, and quantum spin, which dictate chemical reactivity and bonding patterns. Macroscopic observables encompass bulk phenomena such as temperature, pressure, and phase transitions, arising from the collective behavior of vast atomic assemblies. The link between atomic-scale quantum effects and macroscopic thermodynamic properties underpins material science and physical chemistry.
Behavior of Matter at Atomic and Macroscopic Scales
Matter exhibits distinct behaviors at atomic and macroscopic scales, governed by quantum mechanics and classical physics respectively. At the atomic level, particles such as electrons, protons, and neutrons interact through quantum states, energy quantization, and wave-particle duality, leading to phenomena like electron orbitals and chemical bonding. In contrast, macroscopic matter follows classical laws, with properties such as temperature, pressure, and volume emerging from the collective behavior of vast numbers of atoms and molecules, enabling predictable bulk properties like solidity, fluidity, and elasticity.
Measurement Techniques for Each Scale
Atomic-scale measurement techniques utilize tools like scanning tunneling microscopy (STM) and atomic force microscopy (AFM) to resolve individual atoms with sub-nanometer precision, enabling the study of surface structures and atomic interactions. Macroscopic measurement techniques involve instruments such as optical microscopes, laser Doppler vibrometers, and strain gauges, which assess properties and behaviors over millimeter to meter scales in materials and structures. The choice of technique depends on the spatial resolution required, with atomic methods targeting quantum-scale phenomena and macroscopic methods addressing bulk material characteristics.
Real-World Examples: From Atoms to Everyday Objects
Atomic particles such as electrons and protons form the fundamental building blocks of matter, with hydrogen atoms constituting the simplest elements in the universe. In the macroscopic world, these atoms combine into molecules and materials, exemplified by water molecules making up oceans and steel composing skyscraper frameworks. Understanding the transition from atomic interactions to properties of everyday objects enables advancements in nanotechnology, materials science, and engineering applications.
Importance in Science and Technology
Atomic-scale phenomena govern the fundamental properties of matter, influencing chemical reactions, material strength, and electronic behavior, which are crucial for advancements in nanotechnology and quantum computing. Macroscopic understanding allows engineers to design and optimize large-scale systems such as engines, bridges, and electronic devices by applying principles derived from atomic interactions to real-world applications. Bridging atomic and macroscopic perspectives accelerates innovation in fields like material science, semiconductor fabrication, and biomedical engineering, driving technological progress and scientific discovery.
Conclusion: Bridging the Atomic and Macroscopic Worlds
Understanding the connection between atomic and macroscopic scales reveals how atomic interactions govern macroscopic properties such as temperature, pressure, and phase changes. Advancements in nanotechnology and materials science increasingly allow manipulation of atomic structures to design materials with tailored macroscopic behaviors. Bridging these scales enhances predictive modeling, enabling innovations in energy storage, electronics, and biomedical applications.
Atomic Infographic
 libterm.com
libterm.com