Chaperone-mediated autophagy (CMA) is a selective cellular process that targets specific proteins for degradation within lysosomes, maintaining protein quality and cellular homeostasis. This mechanism plays a critical role in preventing the accumulation of damaged or misfolded proteins, which is essential for cellular health and longevity. Discover how CMA influences your cell's function and why it matters by reading the rest of the article.
Table of Comparison
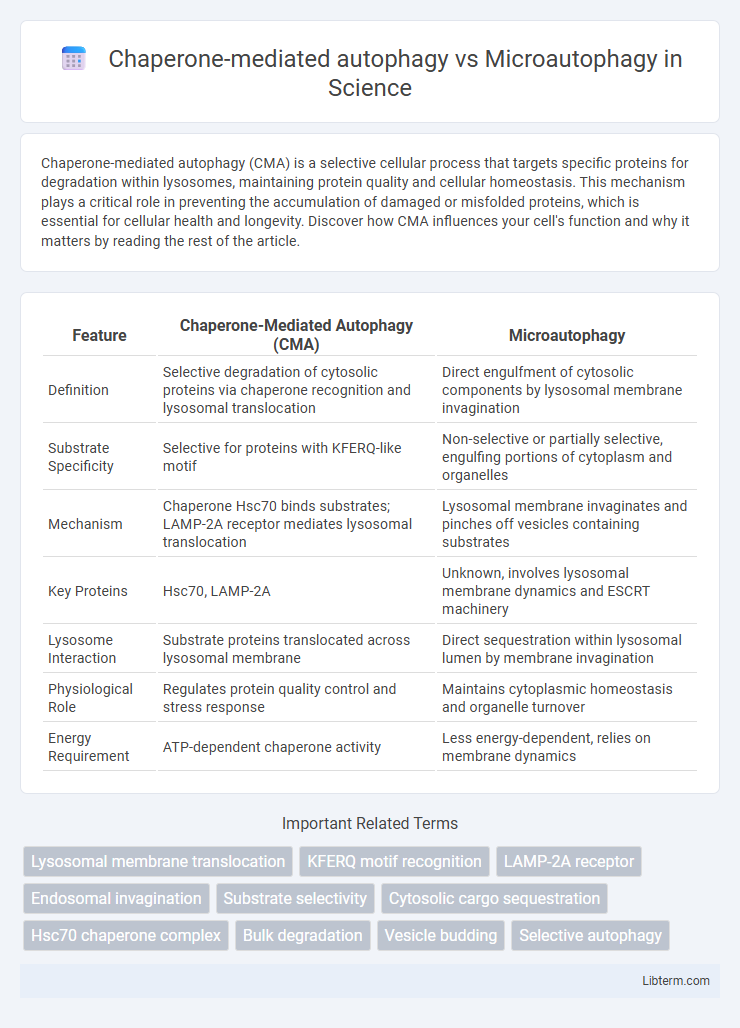
| Feature | Chaperone-Mediated Autophagy (CMA) | Microautophagy |
|---|---|---|
| Definition | Selective degradation of cytosolic proteins via chaperone recognition and lysosomal translocation | Direct engulfment of cytosolic components by lysosomal membrane invagination |
| Substrate Specificity | Selective for proteins with KFERQ-like motif | Non-selective or partially selective, engulfing portions of cytoplasm and organelles |
| Mechanism | Chaperone Hsc70 binds substrates; LAMP-2A receptor mediates lysosomal translocation | Lysosomal membrane invaginates and pinches off vesicles containing substrates |
| Key Proteins | Hsc70, LAMP-2A | Unknown, involves lysosomal membrane dynamics and ESCRT machinery |
| Lysosome Interaction | Substrate proteins translocated across lysosomal membrane | Direct sequestration within lysosomal lumen by membrane invagination |
| Physiological Role | Regulates protein quality control and stress response | Maintains cytoplasmic homeostasis and organelle turnover |
| Energy Requirement | ATP-dependent chaperone activity | Less energy-dependent, relies on membrane dynamics |
Introduction to Autophagy: Key Cellular Recycling Pathways
Chaperone-mediated autophagy (CMA) selectively degrades specific cytosolic proteins by translocating them across the lysosomal membrane via chaperone recognition and LAMP-2A receptor interaction. Microautophagy involves the direct engulfment of cytoplasmic components through lysosomal membrane invagination, enabling bulk degradation of organelles and proteins. Both pathways are essential for cellular homeostasis and recycling, complementing macroautophagy in maintaining proteostasis and responding to cellular stress.
Defining Chaperone-mediated Autophagy (CMA)
Chaperone-mediated autophagy (CMA) is a selective lysosomal degradation pathway that targets specific cytosolic proteins containing a KFERQ-like motif for direct translocation across the lysosomal membrane. CMA relies on the chaperone Hsc70 and lysosomal-associated membrane protein type 2A (LAMP-2A) to recognize and transport substrates, distinguishing it from microautophagy, which involves the non-selective engulfment of cytoplasmic content through lysosomal membrane invagination. The specificity and receptor-mediated transport of CMA allow precise regulation of protein quality control and cellular homeostasis.
Understanding Microautophagy: Basic Mechanism
Microautophagy involves the direct engulfment of cytoplasmic components by lysosomes through membrane invagination, allowing selective or non-selective degradation of cellular material. This process is characterized by lysosomal membrane inward budding, which encloses target substrates into vesicles for subsequent degradation. Understanding microautophagy reveals its essential role in maintaining cellular homeostasis by recycling damaged organelles and proteins independently from chaperone-mediated autophagy.
Molecular Machinery: CMA vs Microautophagy
Chaperone-mediated autophagy (CMA) relies on the Hsc70 chaperone complex to recognize KFERQ-like motifs in substrate proteins, directing them to the lysosomal membrane protein LAMP-2A for selective translocation. Microautophagy involves the direct invagination of the lysosomal membrane to engulf cytosolic components, driven by ESCRT machinery and membrane remodeling proteins such as Vps4 and Atg proteins. The molecular machinery of CMA is highly substrate-specific and dependent on chaperone-mediated targeting, whereas microautophagy employs general membrane dynamics for non-selective cargo sequestration.
Substrate Recognition and Selectivity
Chaperone-mediated autophagy (CMA) selectively targets proteins containing a KFERQ-like motif recognized by cytosolic chaperones such as Hsc70, which deliver substrates directly to lysosomal receptors like LAMP-2A for translocation. Microautophagy involves the non-selective engulfment of cytosolic components through lysosomal membrane invagination without requiring specific substrate motifs or chaperone mediation. The distinct substrate recognition mechanisms differentiate CMA's high selectivity from the broader substrate uptake characteristic of microautophagy.
Regulation and Signaling Pathways
Chaperone-mediated autophagy (CMA) is tightly regulated by the heat shock cognate protein 70 (Hsc70) and lysosome-associated membrane protein type 2A (LAMP-2A), with regulation heavily influenced by signaling pathways such as mTOR and calcineurin that modulate LAMP-2A levels and lysosomal membrane dynamics. Microautophagy involves the direct engulfment of cytosolic components by lysosomal membrane invagination, regulated through the ESCRT machinery and influenced by nutrient-sensing pathways like TORC1, which adjust membrane deformation and vesicle formation. Both autophagy types respond to cellular stress and nutrient status but engage distinct molecular regulators and signaling cascades to maintain proteostasis and adapt cellular metabolism.
Biological Roles and Cellular Functions
Chaperone-mediated autophagy (CMA) selectively degrades cytosolic proteins containing KFERQ-like motifs by directly translocating them into lysosomes, playing a critical role in protein quality control, stress response, and metabolic regulation. In contrast, microautophagy involves the non-selective engulfment of cytoplasmic components through lysosomal membrane invagination, contributing to membrane homeostasis and the turnover of organelles and cytosolic materials. Both pathways maintain cellular homeostasis by regulating protein and organelle degradation, but CMA provides specificity in substrate targeting while microautophagy supports bulk degradation processes.
Implications in Human Diseases
Chaperone-mediated autophagy (CMA) selectively degrades cytosolic proteins bearing KFERQ-like motifs, playing a crucial role in maintaining cellular homeostasis and proteostasis, with dysregulation linked to neurodegenerative diseases such as Parkinson's and Alzheimer's. Microautophagy involves the direct engulfment of cytoplasmic components by lysosomal membrane invagination, contributing to bulk degradation and nutrient recycling; impairments in microautophagy are associated with metabolic disorders and cancer progression. Therapeutic strategies targeting CMA enhancement show promise in mitigating protein aggregation pathologies, while modulation of microautophagy pathways offers potential in controlling tumor growth and metabolic imbalance.
Therapeutic Potential and Research Advances
Chaperone-mediated autophagy (CMA) selectively degrades cytosolic proteins with KFERQ-like motifs, showing promise in treating neurodegenerative diseases by enhancing clearance of pathogenic proteins. Microautophagy engulfs bulk cytoplasmic components directly into lysosomes, offering therapeutic avenues for metabolic disorders through modulation of cellular homeostasis. Recent research advances highlight targeted activation of CMA and microautophagy pathways as innovative strategies to improve cellular quality control and mitigate disease progression.
Future Directions and Unresolved Questions
Future directions in chaperone-mediated autophagy (CMA) research emphasize elucidating the molecular mechanisms governing substrate selectivity and lysosomal translocation, aiming to develop targeted therapies for neurodegenerative diseases. Microautophagy studies seek to clarify the regulatory signaling pathways and the physiological relevance of vesicle-mediated cytosolic material engulfment, with a focus on organelle homeostasis and aging. Unresolved questions include the crosstalk dynamics between CMA and microautophagy under cellular stress and how these pathways compensate or synergize in maintaining proteostasis.
Chaperone-mediated autophagy Infographic
 libterm.com
libterm.com